Label: DIPHENHYDRAMINE HYDROCHLORIDE injection, solution
- NDC Code(s): 67457-124-10
- Packager: Mylan Institutional LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 16, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
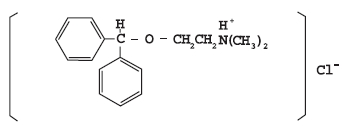
DESCRIPTIONDiphenhydramine hydrochloride is an antihistamine drug having the chemical name 2-(Diphenylmethoxy)-N, N-dimethylethylamine hydrochloride. It occurs as a white, crystalline powder, is freely ...
-
CLINICAL PHARMACOLOGYDiphenhydramine hydrochloride is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector ...
-
INDICATIONS AND USAGEDiphenhydramine hydrochloride injection, is effective in adults and pediatric patients, other than premature infants and neonates, for the following conditions when diphenhydramine hydrochloride ...
-
CONTRAINDICATIONSUse in Neonates or Premature Infants - This drug should not be used in neonates or premature infants. Use in Nursing Mothers - Because of the higher risk of antihistamines for infants ...
-
WARNINGSAntihistamines should be used with considerable caution in patients with narrow-angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy, or ...
-
PRECAUTIONSGeneral - Diphenhydramine hydrochloride has an atropine-like action and, therefore, should be used with caution in patients with a history of bronchial asthma, increased intraocular pressure ...
-
ADVERSE REACTIONSThe most frequent adverse reactions are underscored. 1. General: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose, and ...
-
OVERDOSAGEAntihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in pediatric patients. Atropine-like signs and symptoms; dry ...
-
DOSAGE AND ADMINISTRATIONTHIS PRODUCT IS FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION ONLY. Diphenhydramine hydrochloride injection, is indicated when the oral form is impractical. Parenteral drug products should be ...
-
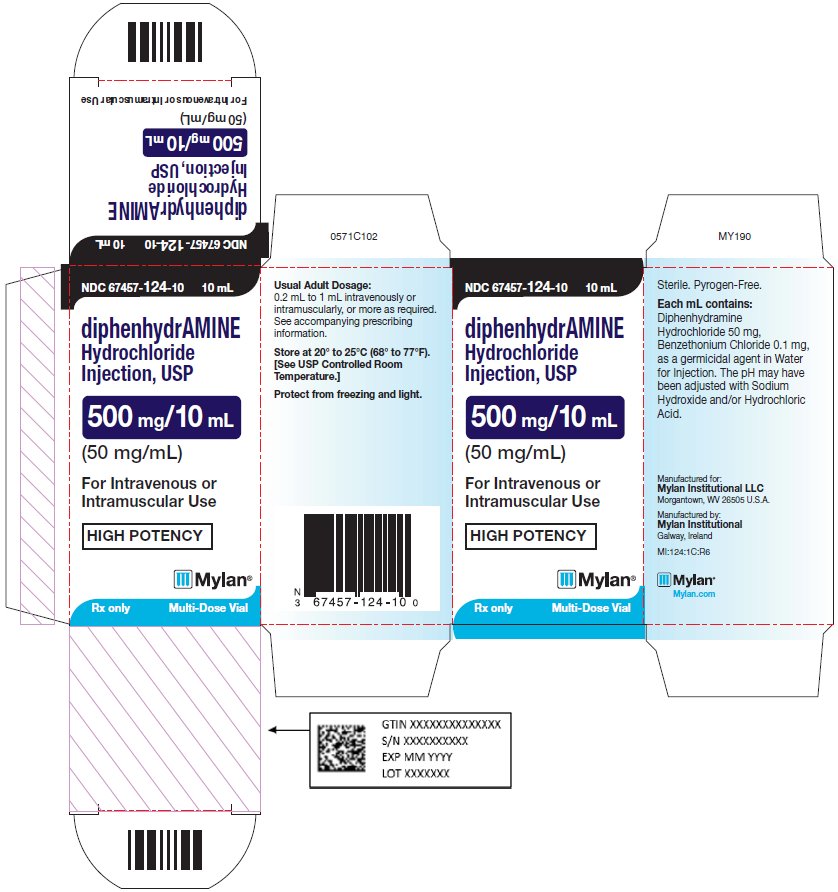
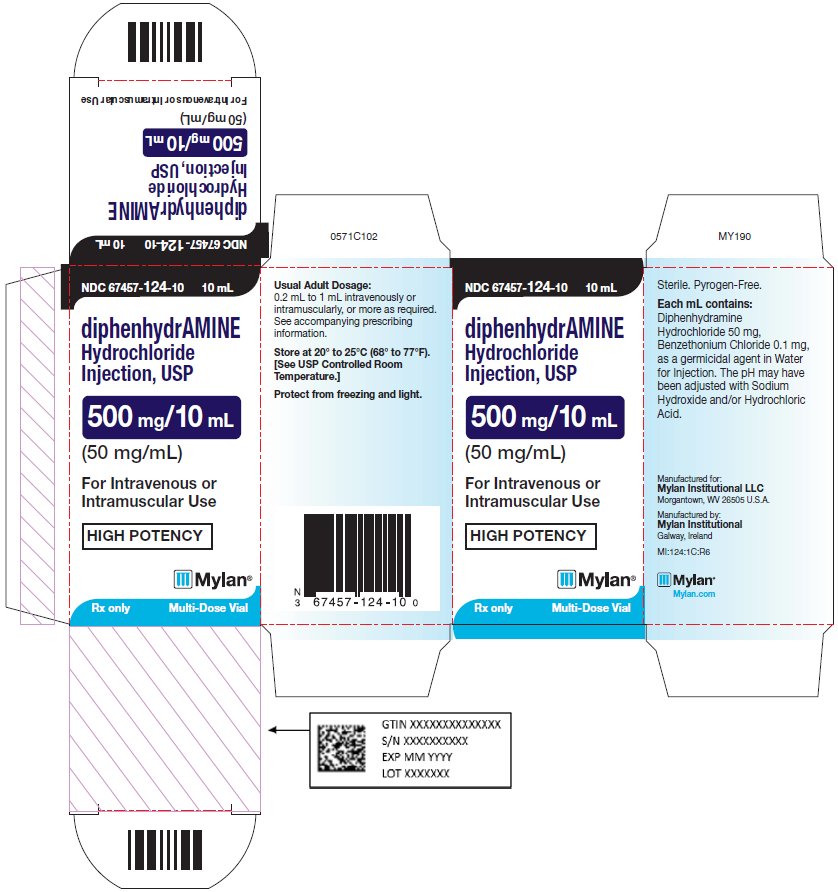
HOW SUPPLIEDDiphenhydramine Hydrochloride Injection, USP is supplied as a sterile solution containing 50 mg diphenhydramine hydrochloride in each milliliter of solution with 0.1 mg/mL benzethonium chloride as ...
-
PRINCIPAL DISPLAY PANEL - 50 mg/mL NDC 67457-124-10 10 mL - diphenhydrAMINE - Hydrochloride - Injection, USP - 500 mg/10 mL - (50 mg/mL) For Intravenous or - Intramuscular Use - HIGH POTENCY - Rx only Multi-Dose ...
-
INGREDIENTS AND APPEARANCEProduct Information