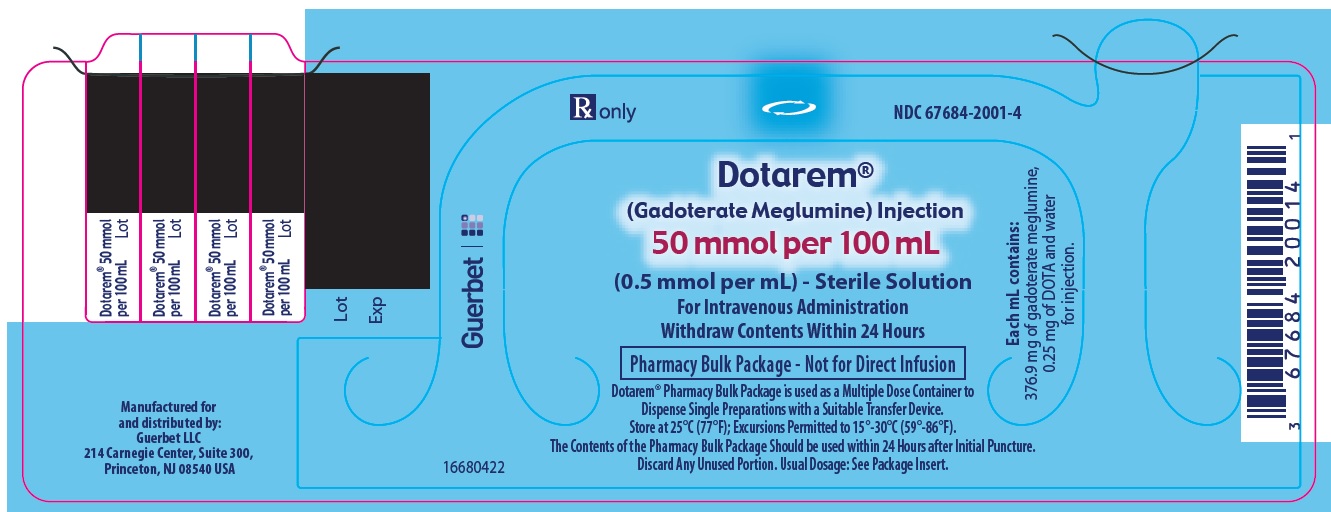
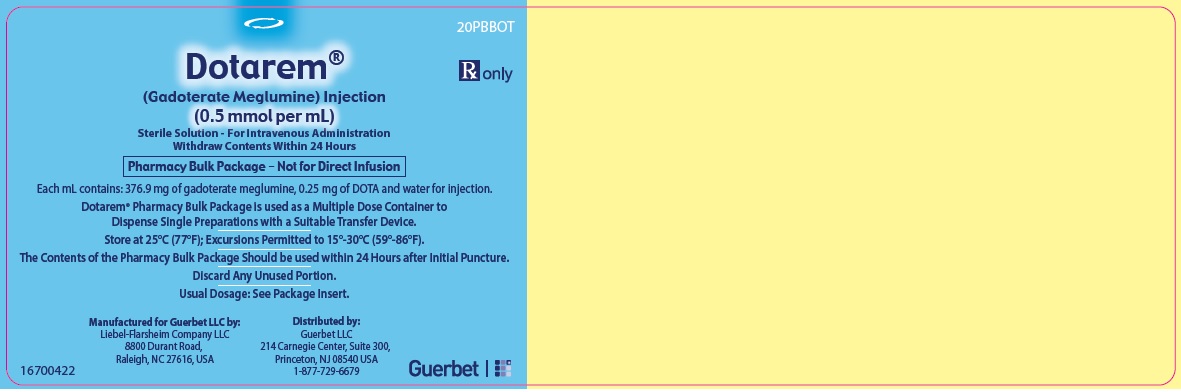
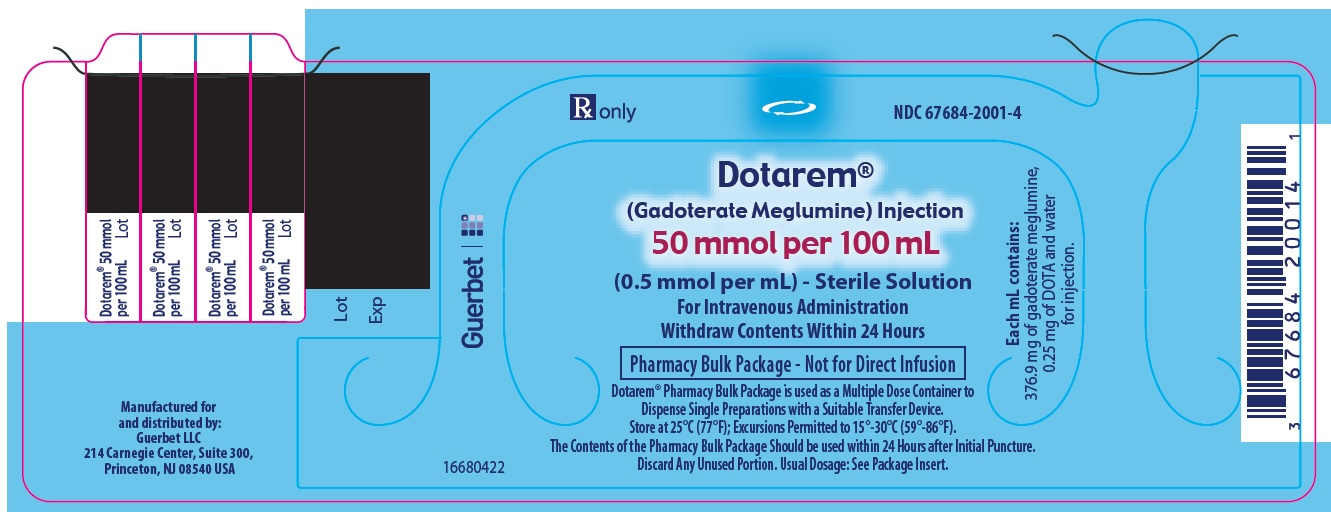
Label: DOTAREM- gadoterate meglumine injection
- NDC Code(s): 67684-2000-4, 67684-2001-4
- Packager: Guerbet LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOTAREM safely and effectively. See full prescribing information for DOTAREM. DOTAREM® (gadoterate meglumine) Injection for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse
reactions including death, coma, encephalopathy, and seizures. DOTAREM is not approved for intrathecal use [see Warnings and Precautions (5.1)].Nephrogenic Systemic Fibrosis
GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of DOTAREM in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.-
The risk for NSF appears highest among patients with:
- Chronic, severe kidney disease (GFR < 30 mL/min/1.73 m2), or
- Acute kidney injury.
Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension, diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing (5.1).
For patients at highest risk for NSF, do not exceed the recommended DOTAREM dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration [see Warnings and Precautions (5.2)].
Close -
The risk for NSF appears highest among patients with:
-
1 INDICATIONS AND USAGEDOTAREM is a gadolinium-based contrast agent indicated for intravenous use with magnetic resonance imaging (MRI) in brain (intracranial), spine and associated tissues in adult and pediatric ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Guidelines - For adult and pediatric patients (including term neonates), the recommended dose of DOTAREM is 0.2 mL/kg (0.1 mmol/kg) body weight administered as an intravenous bolus ...
-
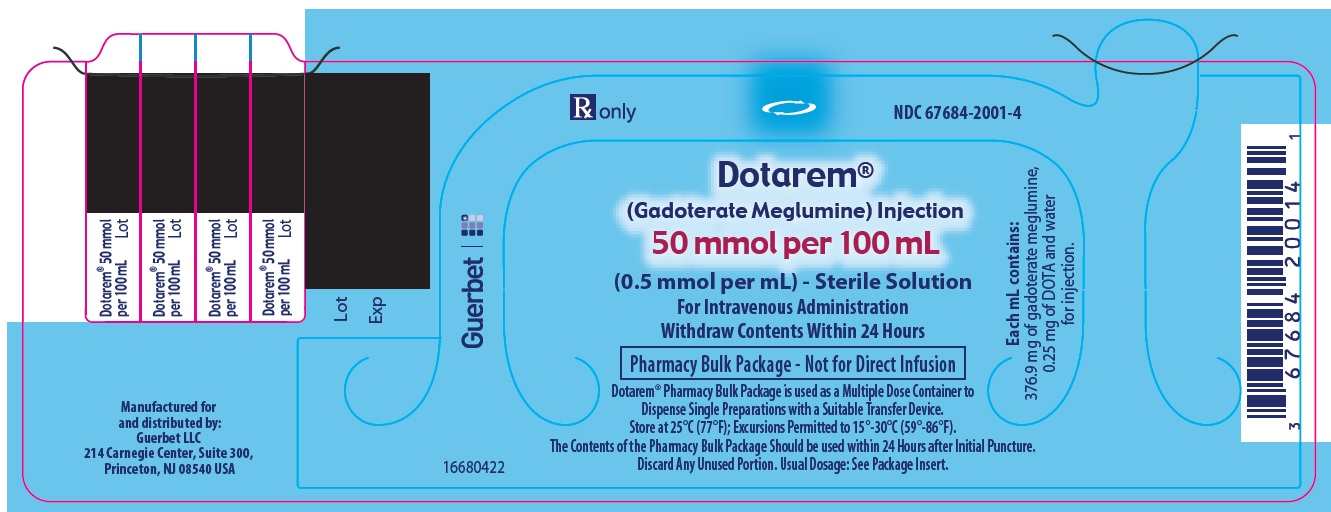
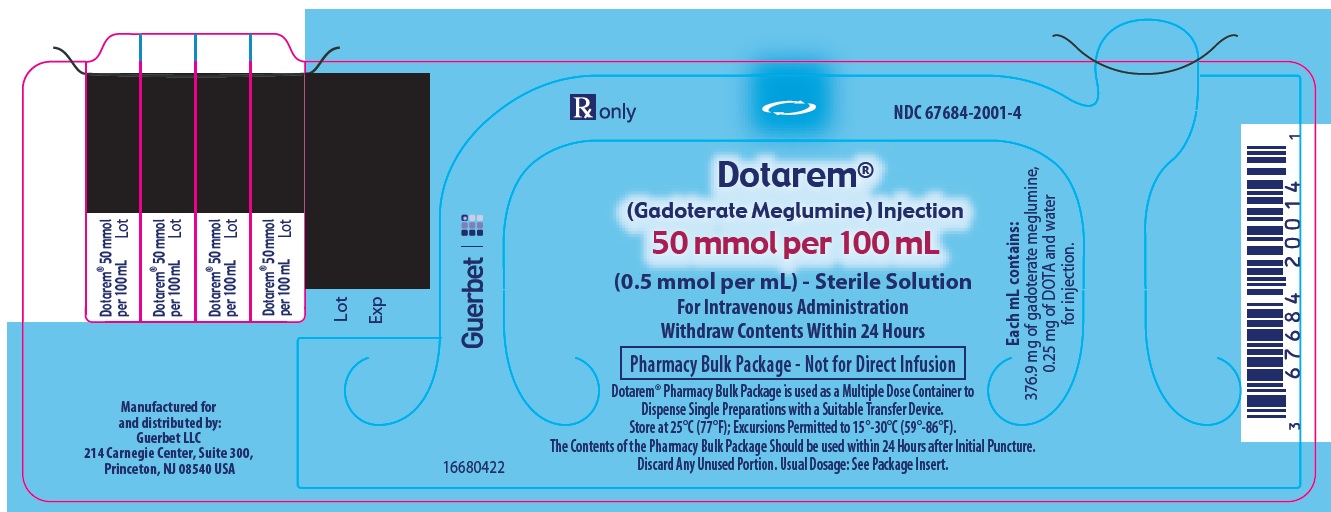
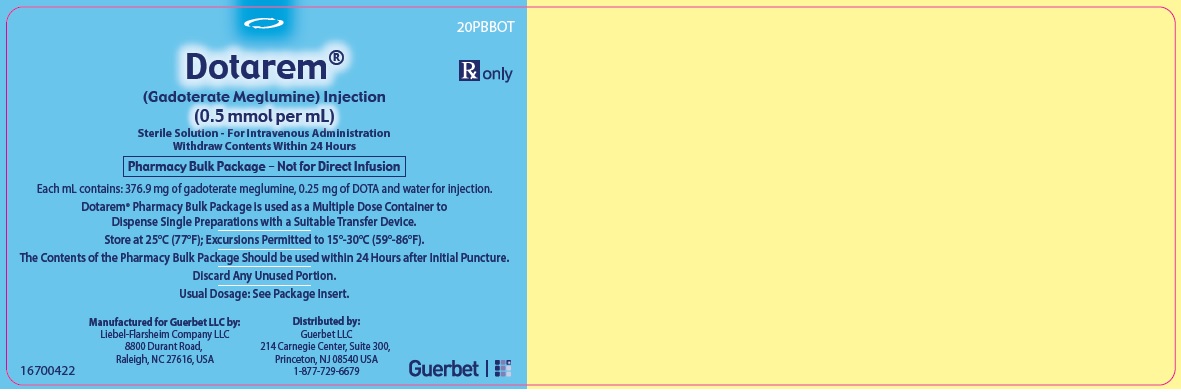
3 DOSAGE FORMS AND STRENGTHSDOTAREM 0.5 mmol/mL is a sterile, clear, colorless to yellow, aqueous solution for intravenous injection containing 376.9 mg/mL gadoterate meglumine. DOTAREM Pharmacy Bulk Package is available in ...
-
4 CONTRAINDICATIONSHistory of clinically important hypersensitivity reactions to DOTAREM [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS5.1 Risk Associated with Intrathecal Use - Intrathecal administration of GBCAs can cause serious adverse reactions including death, coma, encephalopathy, and seizures ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Nephrogenic systemic fibrosis [see Warnings and Precautions (5.2)]. Hypersensitivity ...
-
7 DRUG INTERACTIONSGadoterate does not interfere with serum and plasma calcium measurements determined by colorimetric assays. Specific drug interaction studies with DOTAREM have not been conducted.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - GBCAs cross the human placenta and result in fetal exposure and gadolinium retention. The human data on the association between GBCAs and adverse fetal outcomes ...
-
10 OVERDOSAGEDOTAREM administered to healthy volunteers and to adult patients at cumulative doses up to 0.3 mmol/kg was tolerated in a manner similar to lower doses. Adverse reactions to overdosage with ...
-
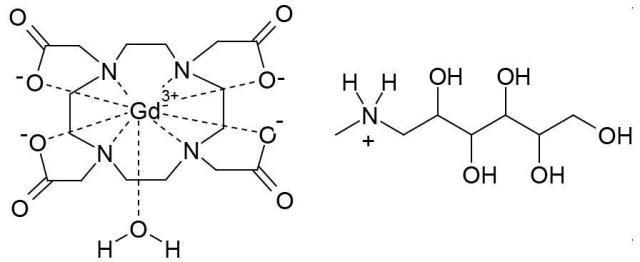
11 DESCRIPTIONDOTAREM (gadoterate meglumine) is a paramagnetic macrocyclic ionic contrast agent administered for magnetic resonance imaging. The chemical name for gadoterate meglumine is D-glucitol ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Gadoterate is a paramagnetic molecule that develops a magnetic moment when placed in a magnetic field. The magnetic moment enhances the relaxation rates of water ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of gadoterate meglumine. Gadoterate meglumine ...
-
14 CLINICAL STUDIES
CNS Imaging - Efficacy and safety of DOTAREM were evaluated in a multi-center clinical trial (Study A) that enrolled 364 adult and 38 pediatric patients (aged ≥ 2 years) with known or suspected ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
DOTAREM Injection is a clear, colorless to yellow solution containing 0.5 mmol/mL of gadoterate meglumine. DOTAREM Injection Pharmacy Bulk Package is supplied in 100 mL vials containing 100 mL ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). 17.1 Nephrogenic Systemic Fibrosis - Instruct patients to inform their healthcare provider if ...
-
Medication GuideDOTAREM® (doh TAH rem) (gadoterate meglumine) Injection for intravenous use - What is the most important information I should know about DOTAREM? GBCAs like DOTAREM may cause serious side ...
-
Principal Package Display
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information