Label: DIPHENHYDRAMINE HYDROCHLORIDE injection
- NDC Code(s): 0641-6282-01, 0641-6282-25
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
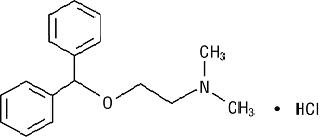
DESCRIPTIONDiphenhydramine Hydrochloride Injection is a sterile, nonpyrogenic solution for intravenous or deep intramuscular use as an antihistaminic agent. Each mL contains diphenhydramine hydrochloride 50 ...
-
CLINICAL PHARMACOLOGYDiphenhydramine hydrochloride is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector ...
-
INDICATIONS AND USAGEDiphenhydramine - Hydrochloride Injection is effective in adults and pediatric patients, other than premature infants and neonates, for the ...
-
CONTRAINDICATIONSUse in Neonates or Premature Infants - This drug - should not be - used in neonates or premature infants. Use in ...
-
WARNINGSAntihistamines - should be used with considerable caution in patients with narrow-angle - glaucoma, stenosing peptic ulcer, pyloroduodenal ...
-
PRECAUTIONSGeneral - Diphenhydramine hydrochloride has an atropine-like action and, therefore, should be used with caution in patients with a ...
-
ADVERSE REACTIONSThe most frequent - adverse reactions are italicized. General - Urticaria; drug rash; anaphylactic shock; photosensitivity ...
-
OVERDOSAGEAntihistamine - overdosage reactions may vary from central nervous system depression to - stimulation. Stimulation is particularly likely in ...
-
DOSAGE AND ADMINISTRATIONTHIS PRODUCT IS FOR - INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION ONLY. Diphenhydramine - Hydrochloride Injection is indicated when the oral ...
-
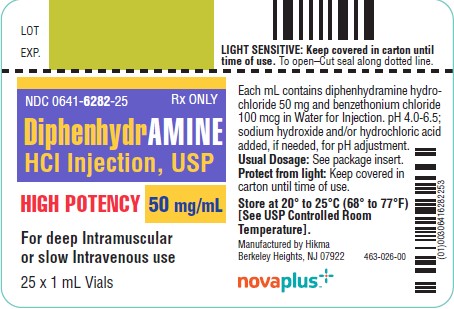
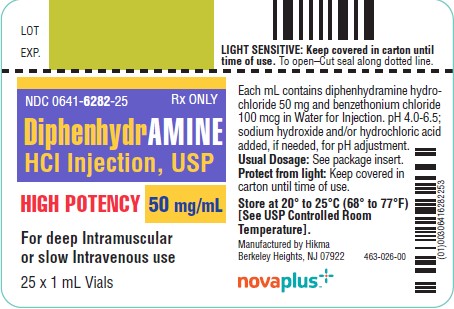
HOW SUPPLIEDDiphenhydramine Hydrochloride Injection, USP 50 mg/mL - 1 mL vials packaged in 25s (NDC 0641-6282-25) Storage - Protect from light. Keep covered in carton until time of use. Store at 20° to 25°C ...
-
PRINCIPAL DISPLAY PANELNDC 0641-6282-01 Rx only - DiphenhydrAMINE - Hydrochloride Injection, USP - 50 mg/mL - HIGH POTENCY - For IM or IV use - Protect from light - 1 mL Vial - NDC 0641-6282-25 Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information