Label: TOPICALE- benzocaine patch
- NDC Code(s): 10733-173-01
- Packager: Medical Products Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Methemoglobinemia warning:
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops pale, grey or blue colored skin (cyanosis), headache, rapid heart rate, shortness of breath, dizziness or lightheadedness, fatigue or lack of energy.
Contraindications:
- Do not use in large quantities or over large areas of body
- Do not use for Teething
- Do not use in children under 2 years of age
Allergy Alert:
Do not use if you have a history of allergy to local anesthetics such as benzocaine, butacaine, procaine or other "caine" anesthetics.
When using this product
Avoid contact with eyes
-
DOSAGE & ADMINISTRATION
- Do not use more than directed.
Adults and children 12 years or older - Apply to the affected area. Allow to remain in place at least one minute and the spit out. Use up to 4 times daily or as directed by a dentist or doctor.
Children 2-12 years of age - Should be supervised in the use of the product
Children under 2 years of age - Do not use
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
INFORMATION FOR OWNERS/CAREGIVERS
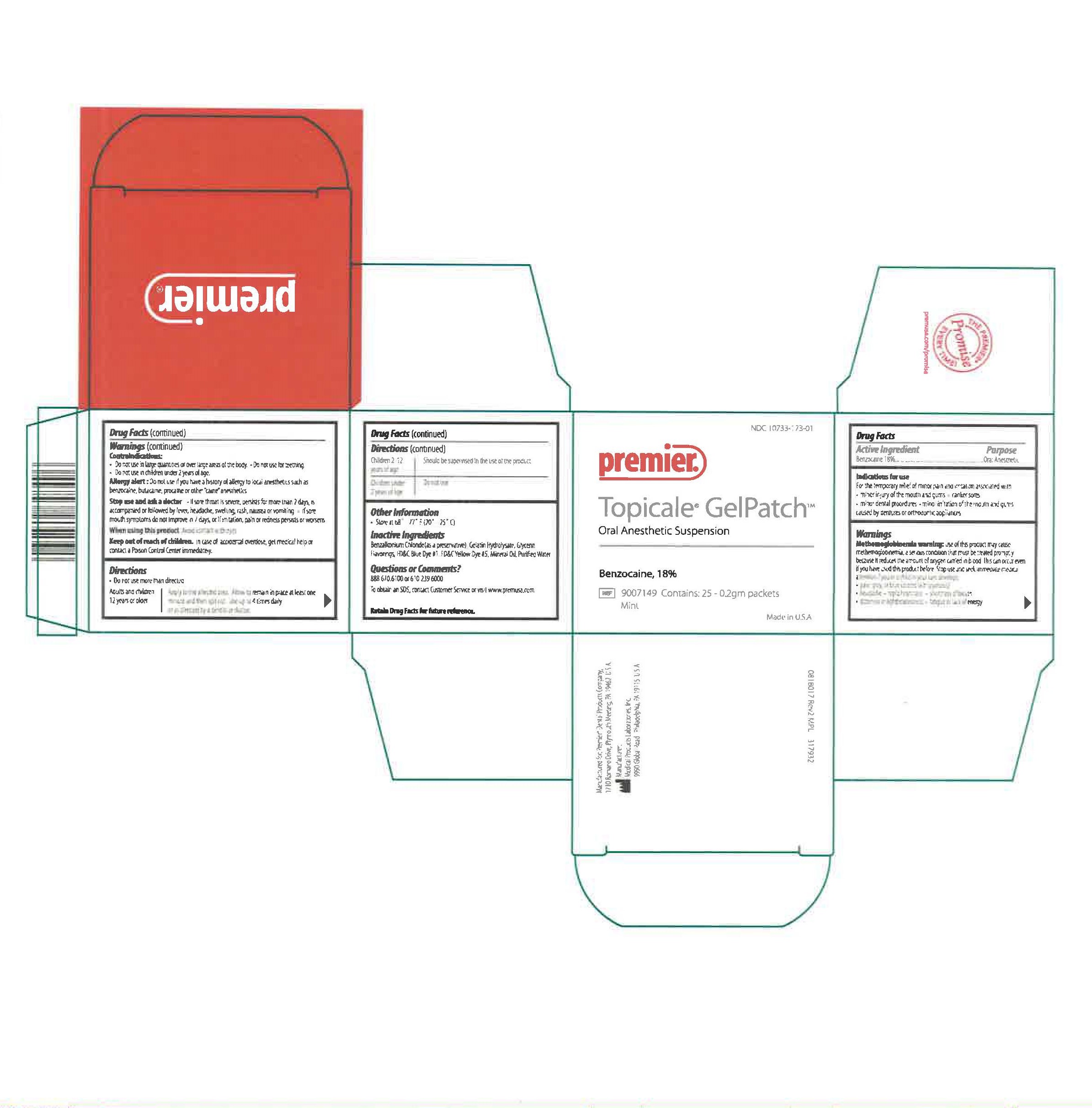
NDC 10733-173-01
Premier
Topicale GelPatch
Oral Anesthetic Suspension
Benzocaine, 18 %
REF 9007149 Contains: 25 - 0.2gm Packets
Mint
Made in U.S.A.
Manufactured for: Premier® Dental Products Company,
1710 Romano Drive, Plymouth Meeting, PA 19462 U.S.A.
Manufacturer: Medical Products Laboratories, Inc.
9990 Global Road Philadelphia, PA 19115 U.S.A.
0818017 Rev2 MPL 317932
Questions or Comments?
888.670.6100 or 610.239.6000
M-Th: 7:30a.m. - 5:30p.m., F: 7:30a.m. - 4:00p.m. EST
To obtain an SDS, contact Customer Service Department or visit www.premusa.com.
Retain drug facts for future reference.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOPICALE
benzocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10733-173 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 180 mg in 1 g Inactive Ingredients Ingredient Name Strength GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10733-173-01 5 g in 1 CARTON; Type 0: Not a Combination Product 05/06/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 05/06/2019 Labeler - Medical Products Laboratories, Inc. (002290302)