Label: ACETAMINOPHEN, ASCORBIC ACID, CHLORPHENIRAMINE MALEATE powder, for solution
- NDC Code(s): 10267-2949-5
- Packager: Contract Pharmacal Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated May 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
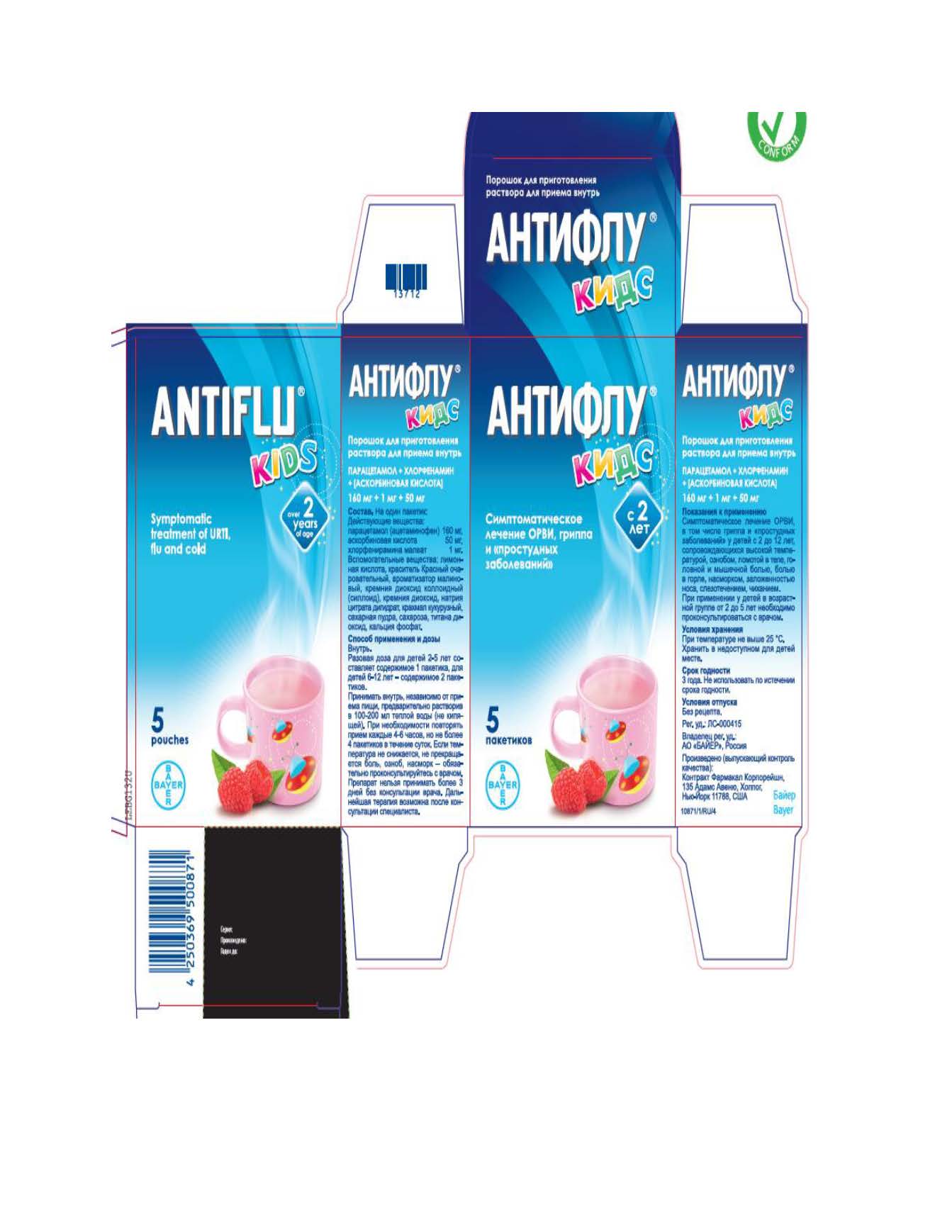
- Official Label (Printer Friendly)
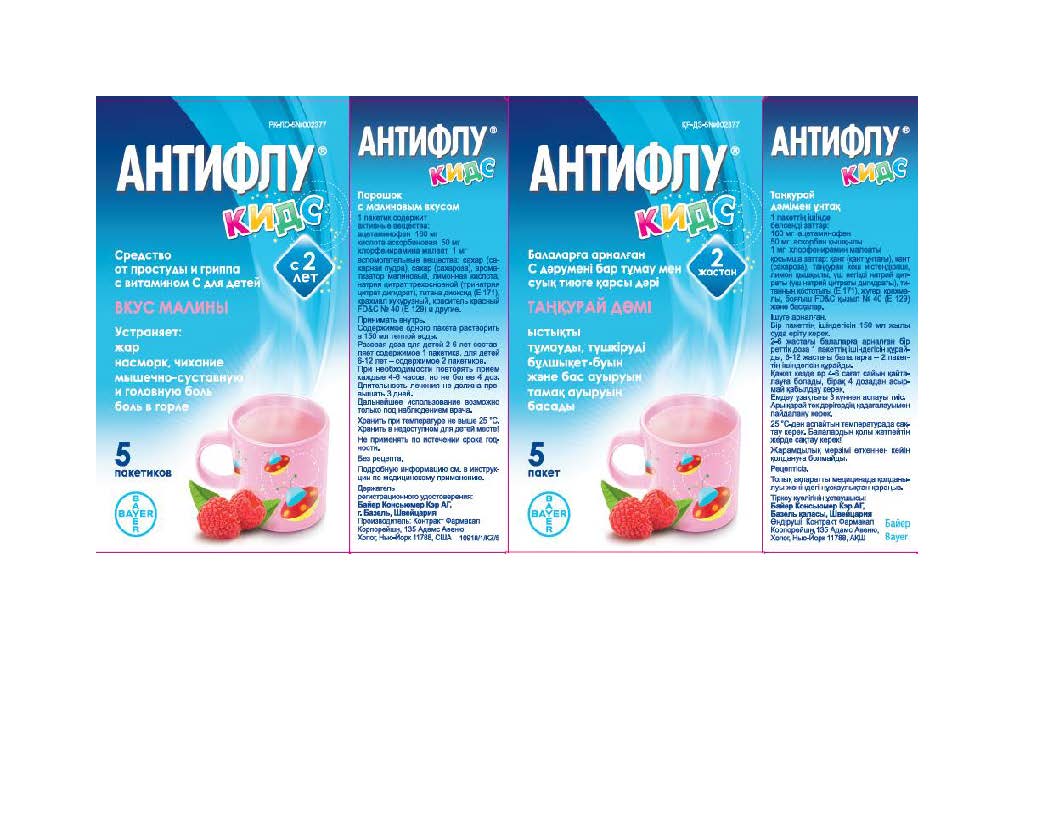
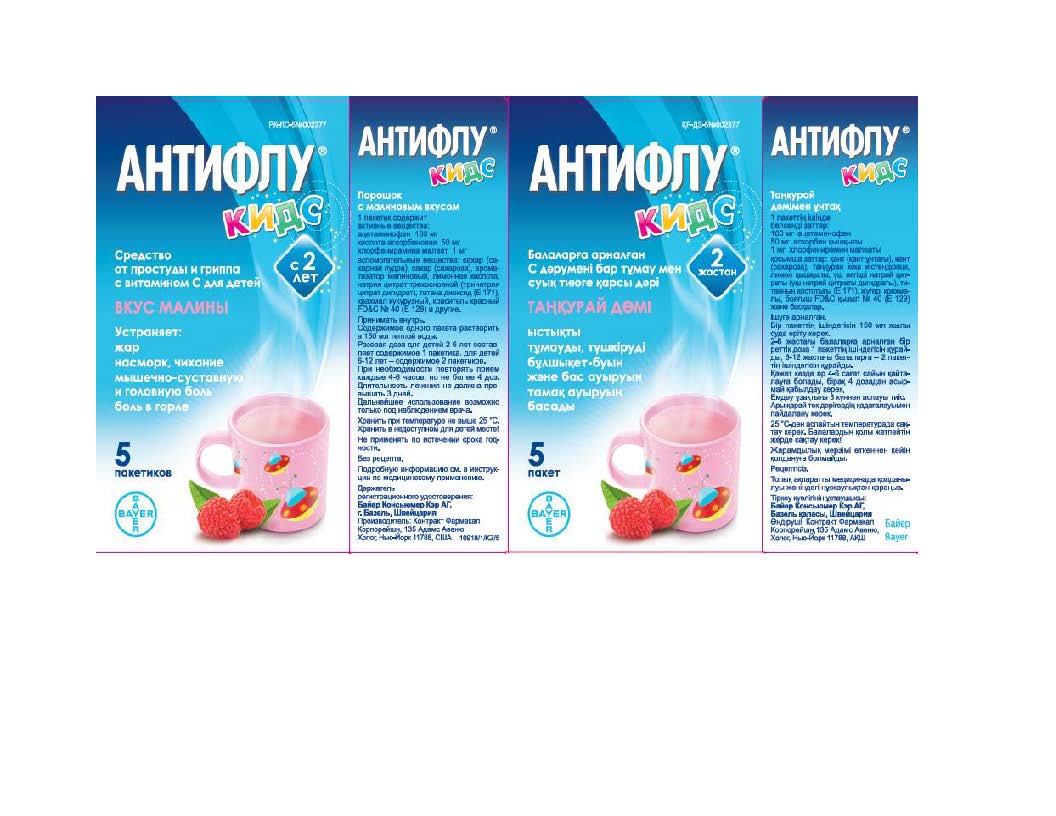
- Belarus Label
- Kazakhstan Label
- Russia Label
- Ukraine Label
- Uzbekistan Label
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN, ASCORBIC ACID, CHLORPHENIRAMINE MALEATE
acetaminophen, ascorbic acid, chlorpheniramine maleate powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10267-2949 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 1 mg in 12000 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 50 mg in 12000 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 12000 mg Inactive Ingredients Ingredient Name Strength RASPBERRY (UNII: 4N14V5R27W) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) FD&C RED NO. 40 (UNII: WZB9127XOA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Product Characteristics Color white Score Shape Size Flavor RASPBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10267-2949-5 5 in 1 CARTON 01/19/2015 1 12000 mg in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/19/2015 Labeler - Contract Pharmacal Corp. (057795122)