Label: MESALAMINE- mesalamine tablet, delayed release
- NDC Code(s): 72603-304-01
- Packager: NorthStar RxLLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MESALAMINE DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information for MESALAMINE DELAYED-RELEASE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMesalamine delayed-release tablets are indicated for the: · induction and maintenance of remission in adult patients with mildly to moderately active ulcerative colitis. · treatment of ...
-
2 DOSAGE AND ADMINISTRATIONAdministration Instructions - • Evaluate renal function prior to initiation of mesalamine delayed-release tablets and periodically while on therapy. • Swallow mesalamine delayed-release ...
-
3 DOSAGE FORMS AND STRENGTHSThe reddish-brown, oval-shaped, enteric-coated tablets containing 1.2 g mesalamine is imprinted with “M19” in black color on one side and plain on other side.
-
4 CONTRAINDICATIONSMesalamine is contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates, or to any of the ingredients of mesalamine - [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Renal Impairment - Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and, rarely, renal failure, has been reported in patients given products ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: • Renal impairment, including renal failure - [see Warnings and Precautions ( 5.1) ...
-
7 DRUG INTERACTIONS7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs - The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Published data from meta-analyses, cohort studies, and case series on the use of mesalamine during pregnancy have not reliably informed an association with ...
-
10 OVERDOSAGEMesalamine is an aminosalicylate, and symptoms of salicylate toxicity may include nausea, vomiting, abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness ...
-
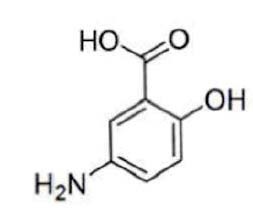
11 DESCRIPTIONEach mesalamine delayed-release tablet, USP for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine), an anti-inflammatory agent. Mesalamine also has the chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of mesalamine is not fully understood, but it appears to have a topical anti-inflammatory effect on the colonic epithelial cells. Mucosal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 104-week dietary carcinogenicity study in CD-1 mice, mesalamine at doses up to 2500 mg/kg/day was not ...
-
14 CLINICAL STUDIES14.1 Adults with Mildly to Moderately Active Ulcerative Colitis - Induction of Remission - Two similarly designed, randomized, double-blind, placebo-controlled trials (Study 1, NCT00503243 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMesalamine delayed-release tablets, USP are available as reddish-brown, oval-shaped, enteric-coated tablets containing 1.2 g mesalamine is imprinted with “M19” in black color on one side and ...
-
17 PATIENT COUNSELING INFORMATIONRenal Impairment - Inform patients that mesalamine delayed-release tablets may decrease their renal function, especially if they have known renal impairment or are taking nephrotoxic drugs ...
-
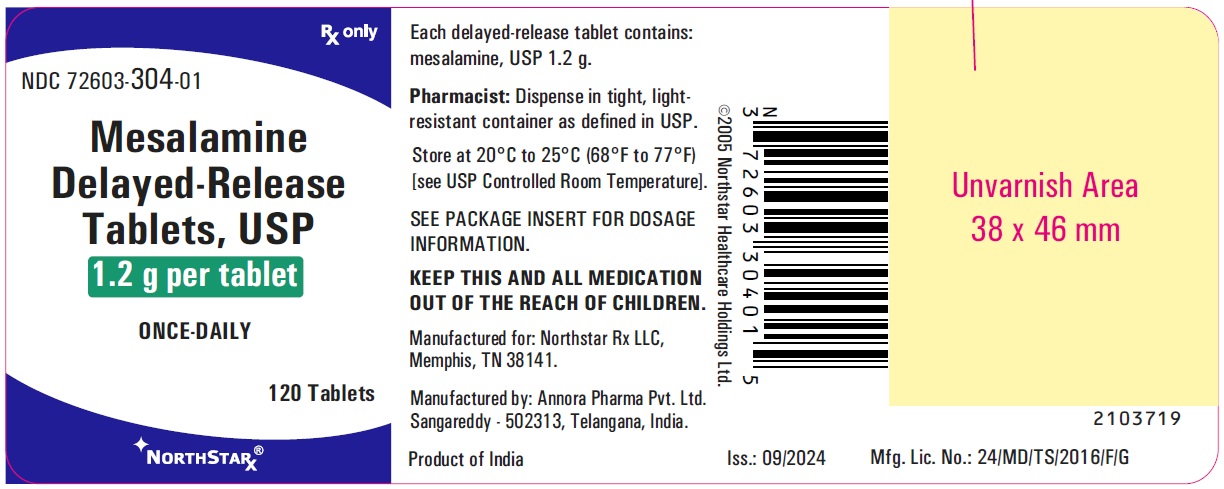
PACKAGE LABEL.PRINCIPAL DISPLAY PANELMesalamine Delayed-Release Tablets, USP 1.2 g - Container Label - 120's count
-
INGREDIENTS AND APPEARANCEProduct Information