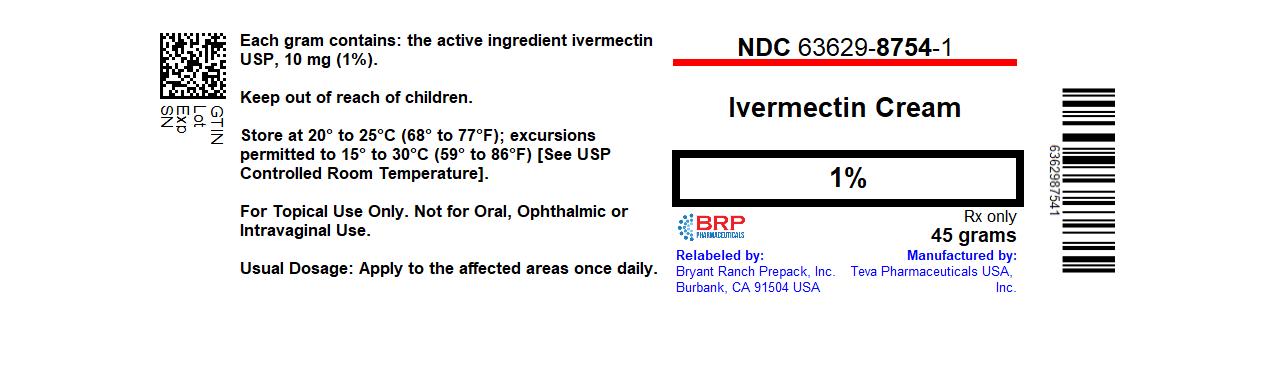
Label: IVERMECTIN cream
- NDC Code(s): 63629-8754-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0591-4052
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IVERMECTIN CREAM safely and effectively. See full prescribing information for IVERMECTIN CREAM.
IVERMECTIN cream, 1%, for topical use
Initial U.S. Approval: 1996INDICATIONS AND USAGE
Ivermectin cream, 1% is indicated for the treatment of inflammatory lesions of rosacea. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Cream, 1%, supplied in tubes of 45 g. (3)
CONTRAINDICATIONS
None. (4)
ADVERSE REACTIONS
In controlled clinical trials with ivermectin cream the most common adverse reactions (incidence less than or equal to 1 %) included skin burning sensation and skin irritation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-866-832-8537 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During clinical trials, 2,047 subjects with inflammatory lesions of rosacea received ivermectin cream once daily. A total of 1,555 subjects were treated once daily for more than 12 weeks, and 519 for approximately one year.
Adverse reactions, reported in less than or equal to 1% of subjects treated with ivermectin cream for at least 3 months in vehicle-controlled clinical trials, included skin burning sensation and skin irritation.
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Local adverse reactions: contact dermatitis and allergic dermatitis.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. Ivermectin cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Note: The animal multiples of human exposure calculations were based on AUC comparisons. The maximum topical human dose (MTHD) of ivermectin cream is 1 g applied once daily.
Systemic embryofetal development studies were conducted in rats and rabbits. Oral doses of 1.5 mg/kg/day, 4 mg/kg/day, and 12 mg/kg/day ivermectin were administered during the period of organogenesis (gestational days 6 to 17) to pregnant female rats. Maternal death occurred at 12 mg/kg/day (1909X MTHD). Cleft palate occurred in the fetuses from the 12 mg/kg/day (1909X MTHD) group. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 4 mg/kg/day (708X MTHD). Oral doses of 0.5 mg/kg/day, 1.5 mg/kg/day, 2.5 mg/kg/day, 3.5 mg/kg/day, and 4.5 mg/kg/day ivermectin were administered during the period of organogenesis (gestational days 7 to 20) to pregnant female rabbits. Maternal death occurred at doses greater than or equal to 2.5 mg/kg/day (72X MTHD). Carpal flexure occurred in the fetuses from the 4.5 mg/kg/day (354X MTHD) group. Fetal weight decrease was noted at 3.5 mg/kg/day (146X MTHD). No treatment related effects on embryofetal toxicity were noted at 2.5 mg/kg/day (72X MTHD) and no treatment related effects on teratogenicity were noted at 3.5 mg/kg/day (146X MTHD).
A pre- and post-natal development study was conducted in rats. Oral doses of 1 mg/kg/day, 2 mg/kg/day, and 4 mg/kg/day ivermectin were administered to pregnant female rats during gestational days 6 to 20 and lactation days 2 to 20. Neonatal death occurred at doses greater than or equal to 2 mg/kg/day. Behavior development of newborn rats was adversely affected at all doses.
8.3 Nursing Mothers
Following oral administration, ivermectin is excreted in human milk in low concentrations. Excretion in human milk following topical administration has not been evaluated. In oral studies in rats, ivermectin was excreted in the milk of nursing mothers and neonatal toxicity was observed in the litters. The blood-brain barrier in neonatal rats may not be fully developed at birth. Because of the potential for serious adverse reactions from ivermectin cream in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of ivermectin cream in pediatric patients have not been established.
8.5 Geriatric Use
Of the 1,371 subjects in the two pivotal clinical studies of ivermectin cream, 170 (12.4%) were 65 and over, while 37 (2.7%) were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
10 OVERDOSAGE
In accidental or significant exposure to unknown quantities of veterinary formulations of ivermectin in humans, either by ingestion, inhalation, injection, or exposure to body surfaces, the following adverse effects have been reported most frequently: rash, edema, headache, dizziness, asthenia, nausea, vomiting, and diarrhea. Other adverse effects that have been reported include: seizure, ataxia, dyspnea, abdominal pain, paresthesia, urticaria, and contact dermatitis.
In case of accidental ingestion, supportive therapy, if indicated, should include parenteral fluids and electrolytes, respiratory support (oxygen and mechanical ventilation if necessary) and pressor agents if clinically significant hypotension is present. Induction of emesis and/or gastric lavage as soon as possible, followed by purgatives and other routine anti-poison measures, may be indicated if needed to prevent absorption of ingested material.
-
11 DESCRIPTION
Ivermectin cream, 1% is a white to pale yellow hydrophilic cream. Each gram of ivermectin cream contains 10 mg of ivermectin, USP. It is intended for topical use.
Ivermectin, USP is a semi-synthetic derivative isolated from the fermentation of Streptomyces avermitilis that belongs to the avermectin family of macrocyclic lactones.
Ivermectin, USP is a mixture containing not less than 95.0 % and not more than 102.0 % of 5-O-demethyl-22,23-dihydroavermectin A1a plus 5-O-demethyl-25-de(1-methylpropyl)-25-(1-methylethyl)-22,23-dihydroavermectin A1a, generally referred to as 22,23-dihydroavermectin B1a and B1b or H2B1a and H2B1b, respectively; and the ratio (calculated by area percentage) of component H2B1a/(H2B1a + H2B1b)) is not less than 90.0 %.
The respective molecular formulas of H2B1a and H2B1b are C48H74O14 and C47H72O14 with molecular weights of 875.10 and 861.07 respectively.
The structural formulas are:
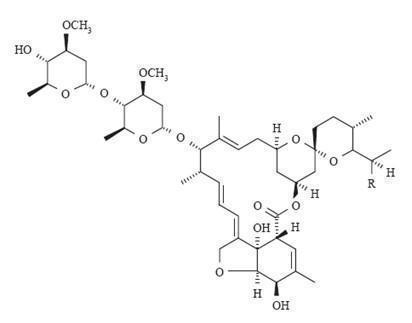
Component H2B1a: R = C2H5, Component H2B1b: R = CH3.
Ivermectin cream, 1% contains the following inactive ingredients: benzyl alcohol, citric acid anhydrous, carbomer homopolymer type c, di-isopropyl adipate, edetate disodium, hexylene glycol, methylparaben, oleyl alcohol, polysorbate 80, propylparaben, purified water, sodium citrate, sodium hydroxide, and sorbitan tristearate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of ivermectin cream in treating rosacea lesions is unknown.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At therapeutic doses, ivermectin cream is not expected to prolong QTc interval.
12.3 Pharmacokinetics
Absorption
The absorption of ivermectin from ivermectin cream was evaluated in a clinical trial in 15 adult male and female subjects with severe papulopustular rosacea applying 1 g ivermectin cream, 1% once daily. At steady state (after 2 weeks of treatment), the highest mean ± standard deviation) plasma concentrations of ivermectin peaked (Tmax) at 10 ± 8 hours post-dose, the maximum concentration (Cmax) was 2.10 ± 1.04 ng/mL (range: 0.69 to 4.02 ng/mL) and the area under the concentration curve (AUC0-24hr) was 36.14 ± 15.56 ng.hr/mL (range: 13.69 to 75.16 ng•hr/mL). In addition, systemic exposure assessment in longer treatment duration (Phase 3 studies) showed that there was no plasma accumulation of ivermectin over the 52-week treatment period.
Distribution
An in vitro study demonstrated that ivermectin is greater than 99% bound to plasma proteins and is bound primarily to human serum albumin. No significant binding of ivermectin to erythrocytes was observed.
Metabolism
In vitro studies using human hepatic microsomes and recombinant CYP450 enzymes have shown that ivermectin is primarily metabolized by CYP3A4. In vitro studies show that ivermectin at therapeutic concentrations does not inhibit the CYP450 isoenzymes 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4 or 4A11, or induce 1A2, 2B6, 2C9 or 3A4.
Excretion
The apparent terminal half-life averaged 6.5 days (mean ± standard deviation: 155± 40 hours, range 92 to 238 hours) in patients receiving a once daily cutaneous application of ivermectin cream for 28 days.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year dermal mouse carcinogenicity study, ivermectin was administered to CD-1 mice at topical doses of 1, 3, and 10 mg/kg/day (0.1%, 0.3% and 1% ivermectin cream applied at 2 mL/kg/day). No drug-related tumors were noted in this study up to the highest dose evaluated in this study of 10 mg/kg/day (747X maximum topical human dose (MTHD)).
In a 2-year oral rat carcinogenicity study, ivermectin was administered to Wistar rats at gavage doses of 1 mg/kg/day, 3 mg/kg/day, and 9 mg/kg/day. A statistically significant increase in the incidence of hepatocellular adenoma was noted in males treated with 9 mg/kg/day (1766X MTHD) ivermectin. The clinical relevance of this finding is unknown. No drug-related tumors were noted in females up to the highest dose evaluated in this study of 9 mg/kg/day (1959X MTHD). No drug-related tumors were noted in males at doses less than or equal to 3 mg/kg/day (599X MTHD).
Ivermectin revealed no evidence of genotoxic potential based on the results of two in vitro genotoxicity tests (the Ames test and the L5178Y/TK+/- mouse lymphoma assay) and one in vivo genotoxicity test (rat micronucleus assay).
In a fertility study, oral doses of 0.1 mg/kg/day, 1 mg/kg/day, and 9 mg/kg/day ivermectin were administered to male and female rats. Mortality occurred at 9 mg/kg/day (1027X MTHD). The precoital period was generally prolonged at 9 mg/kg/day. No treatment related effects on fertility or mating performance were noted at doses less than or equal to 1 mg/kg/day (68X MTHD).
-
14 CLINICAL STUDIES
Ivermectin cream applied once daily at bedtime was evaluated in the treatment of inflammatory lesions of rosacea in two randomized, double-blind, vehicle-controlled clinical trials, which were identical in design. The trials were conducted in 1,371 subjects aged 18 years and older who were treated once daily for 12 weeks with either ivermectin cream or vehicle cream.
Overall, 96% of subjects were Caucasian and 67% were female. Using the 5-point Investigator Global Assessment (IGA) scale (0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe), 79% of subjects were scored as moderate (IGA=3) and 21% scored as severe (IGA=4) at baseline.
The co-primary efficacy endpoints in both pivotal trials were the success rate based on the IGA outcome (percentage of subjects “clear” and “almost clear”) and absolute change from baseline in inflammatory lesion counts at Week 12. Table 1 presents the co-primary efficacy results at Week 12. Ivermectin cream was more effective than vehicle cream on the co-primary efficacy endpoints starting from 4 weeks of treatment in both studies, see Figures 1 through 4.
Table 1: Co-Primary Efficacy Results at Week 12 Study 1
Study 2
Ivermectin
Vehicle
Ivermectin
Vehicle
Cream (N=451)
Cream (N=232)
Cream (N=459)
Cream (N=229)
Investigator Global Assessment:
Number (%) of Subjects Clear or Almost Clear
173 (38.4%)
27 (11.6%)
184 (40.1%)
43 (18.8%)
Inflammatory Lesion Counts:
Mean Absolute (%) Change from Baseline
20.5 (64.9%)
12.0 (41.6%)
22.2 (65.7%)
13.4 (43.4%)
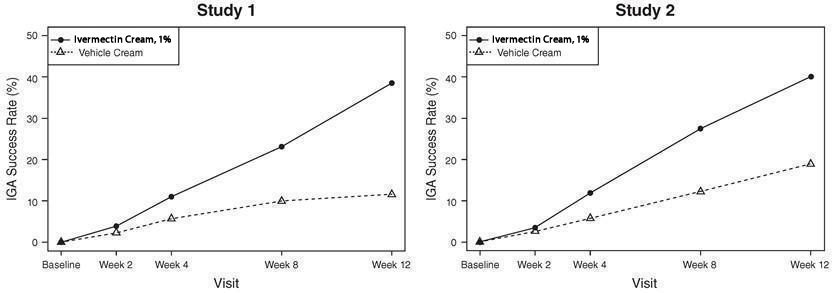
Figures 1 and 2: IGA Success Rates Over Time
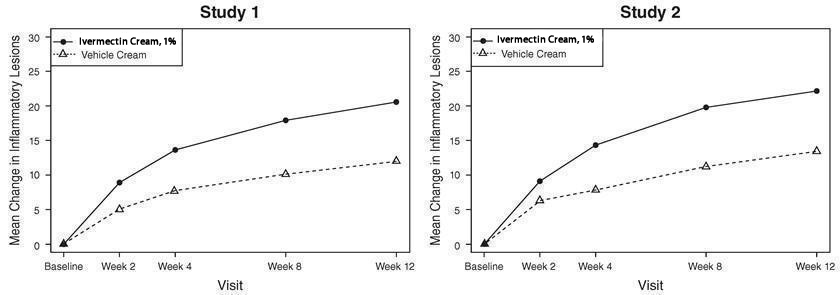
Figures 3 and 4: Mean Absolute Change in Inflammatory Lesion Counts from Baseline Over Time
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Ivermectin cream, 1% is a white to pale yellow cream, supplied in a laminated tube with a child resistant cap in the following size:
45 gram – NDC: 63629-8754-1
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP Controlled Room Temperature].
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
- 17 PATIENT COUNSELING INFORMATION
-
INSTRUCTION FOR USE
Ivermectin (eye" ver mek' tin) Cream, 1%
Important: Ivermectin cream is for use on the face only. Do not use ivermectin cream in your eyes, mouth or vagina.
Read and follow the steps below so that you use ivermectin cream correctly:
1. Open the tube of ivermectin cream by gently pressing down on the child resistant cap and twist in the direction of the arrow (counterclockwise) as shown below. See Figures A and B. To avoid spilling, do not squeeze the tube while opening or closing.
Figure A
Figure B
2. To apply ivermectin cream to your face, squeeze a pea-sized amount of ivermectin cream from the tube onto your fingertip. See Figure C.
Figure C
3. Apply ivermectin to the affected areas of your face once a day. Use a pea-sized amount of ivermectin cream for each area of your face (forehead, chin, nose, each cheek) that is affected. Spread the cream smoothly and evenly in a thin layer. Avoid contact with your eyes and lips.
4. To close ivermectin cream, gently press down on the child resistant cap and twist to the right (clockwise). See Figure D.
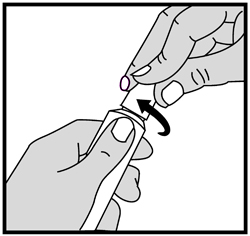
Figure D
How should I store ivermectin cream?
- Store ivermectin cream at room temperature between 68°F to 77°F (20°C to 25°C).
Keep ivermectin cream out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Revised – January 2019
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IVERMECTIN
ivermectin creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63629-8754(NDC:0591-4052) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 10 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) HEXYLENE GLYCOL (UNII: KEH0A3F75J) METHYLPARABEN (UNII: A2I8C7HI9T) OLEYL ALCOHOL (UNII: 172F2WN8DV) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN TRISTEARATE (UNII: 6LUM696811) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-8754-1 1 in 1 CARTON 08/20/2021 1 45 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210019 10/14/2019 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-8754) , RELABEL(63629-8754)