Label: ADVATE (antihemophilic factor- recombinant kit
- NDC Code(s): 0944-2941-01, 0944-2942-02, 0944-2943-02, 0944-2944-02, view more
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADVATE® safely and effectively. See full prescribing information for ADVATE. ADVATE [antihemophilic factor (recombinant) ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEADVATE® [Antihemophilic Factor (Recombinant)] is a recombinant antihemophilic factor indicated for use in children and adults with hemophilia A (congenital factor VIII deficiency) for: Control ...
-
2 DOSAGE AND ADMINISTRATIONFor intravenous injection after reconstitution only. 2.1 Dose - Dosage and duration of treatment depend on the severity of factor VIII deficiency, the location and extent of the bleeding, and ...
-
3 DOSAGE FORMS AND STRENGTHSADVATE is available as a lyophilized white to off-white powder in single-dose vials containing nominally 250, 500, 1000, 1500, 2000, 3000, or 4000 International Units (IU, unit). The 250 to 1500 ...
-
4 CONTRAINDICATIONSADVATE is contraindicated in patients who have life-threatening hypersensitivity reactions, including anaphylaxis, to mouse or hamster protein or other constituents of the product (mannitol ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with ADVATE. Symptoms include dizziness, paresthesia, rash, flushing, facial ...
-
6 ADVERSE REACTIONSSerious adverse reactions seen with ADVATE are hypersensitivity reactions, including anaphylaxis, and the development of high-titer inhibitors necessitating alternative treatments to factor ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data with ADVATE use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with ADVATE. It is not ...
-
11 DESCRIPTIONADVATE [Antihemophilic Factor (Recombinant)] is a purified glycoprotein consisting of 2,332 amino acids that is synthesized by a genetically engineered Chinese hamster ovary (CHO) cell line but ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - ADVATE temporarily replaces the missing coagulation factor VIII that is needed for effective hemostasis. 12.2 Pharmacodynamics - The activated partial thromboplastin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted with the active ingredient in ADVATE to assess its mutagenic or carcinogenic potential. RECOMBINATE was ...
-
14 CLINICAL STUDIESOriginal Safety and Efficacy Study - A safety and efficacy trial evaluated the pharmacokinetics (double-blinded, randomized, crossover), safety, immunogenicity, and hemostatic efficacy ...
-
15 REFERENCESFischer K, Collins P, Björkman S, Blanchette V, Oh M, Fritsch S, Schroth P, Spotts G, Ewenstein B. Trends in bleeding patterns during prophylaxis for severe haemophilia: observations from a ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ADVATE in a BAXJECT III system is packaged with 2 mL or 5 mL of Sterile Water for Injection, one Terumo Microbore Infusion set (2 mL only), one full prescribing physician insert ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Advise patients to report any adverse reactions or problems following ADVATE ...
-
SPL UNCLASSIFIED SECTIONTakeda Pharmaceuticals U.S.A., Inc. Cambridge, MA 02142 - U.S. License No. 1898 - ADVATE, BAXJECT and RECOMBINATE are registered trademarks of Baxalta Incorporated. Takeda and are registered ...
-
Patient InformationADVATE® (ad-vate) [Antihemophilic Factor (Recombinant)] This leaflet summarizes important information about ADVATE. Please read it carefully before using this medicine. This information does not ...
-
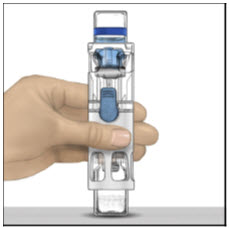
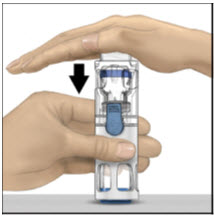
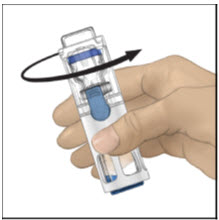
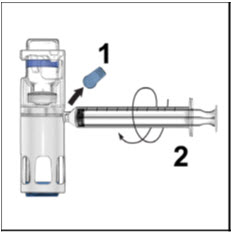
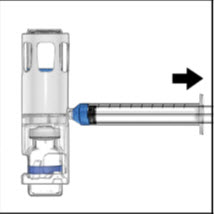
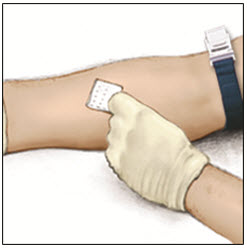
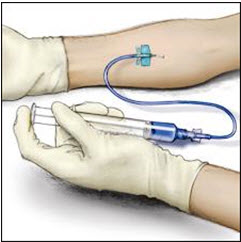
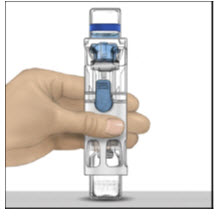
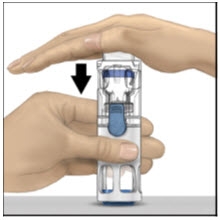
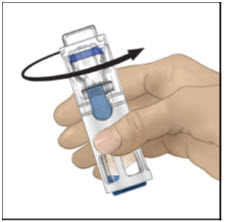
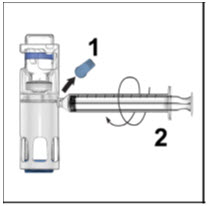
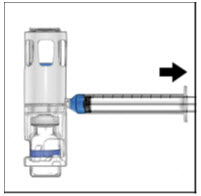
Instructions For UseADVATE® [Antihemophilic Factor (Recombinant)] (For intravenous use only) Do not attempt to do an infusion to yourself unless you have been taught how by your healthcare provider or hemophilia ...
-
PRINCIPAL DISPLAY PANEL - 250 IU 2 mL Kit CartonADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3051-02 - Single-dose only - For Intravenous Administration Only - Rx Only - Lyophilized powder for reconstitution - ADVATE and diluent ...
-
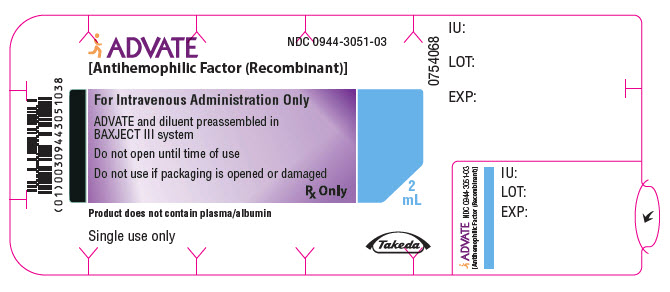
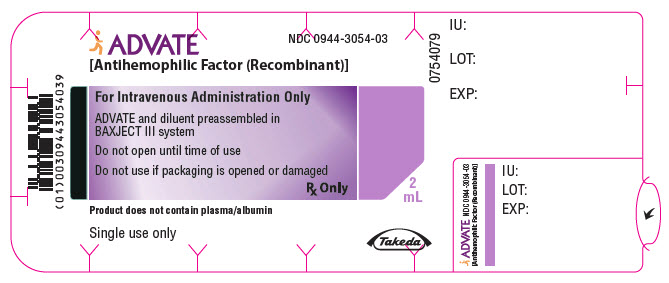
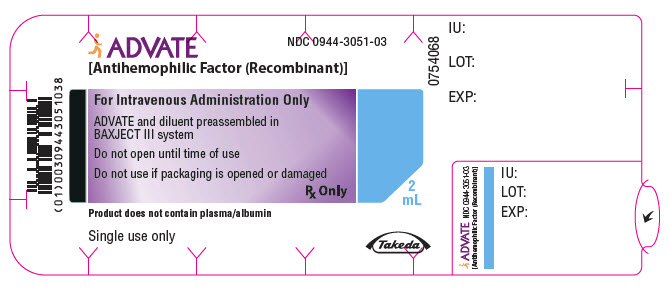
PRINCIPAL DISPLAY PANEL - 250 IU 2 mL Blister Pack LabelADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3051-03 - For Intravenous Administration Only - ADVATE and diluent preassembled in - BAXJECT® III system - Do not open until time of use - Do not ...
-
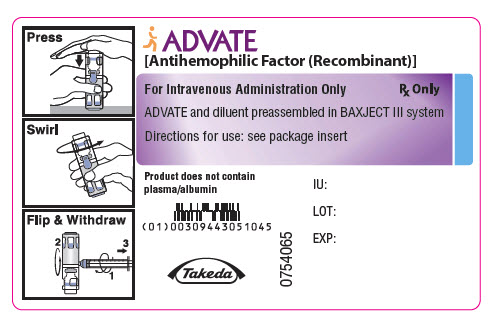
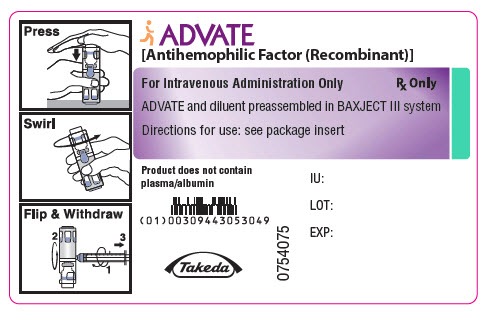
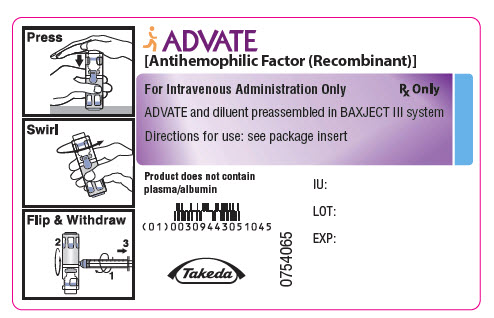
PRINCIPAL DISPLAY PANEL - 250 IU 2 mL Container LabelADVATE® [Antihemophilic Factor (Recombinant)] For Intravenous Administration Only - Rx Only - ADVATE and diluent preassembled in BAXJECT® III system - Directions for use: see package ...
-
PRINCIPAL DISPLAY PANEL - 500 IU 2 mL Kit CartonADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3052-02 - Single-dose only - For Intravenous Administration Only - Rx Only - Lyophilized powder for reconstitution - ADVATE and diluent ...
-
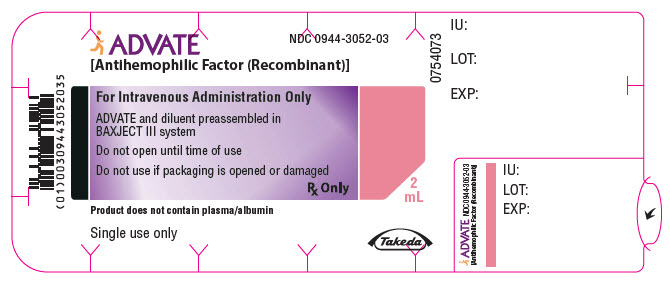
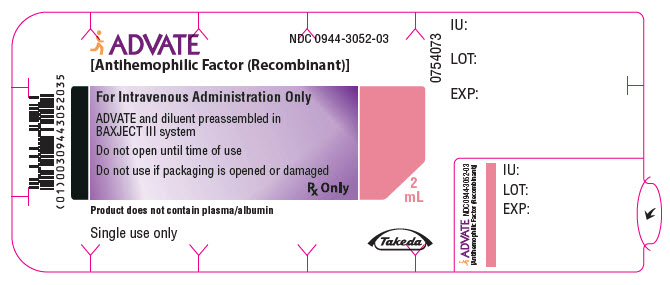
PRINCIPAL DISPLAY PANEL - 500 IU 2 mL Blister Pack LabelADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3052-03 - For Intravenous Administration Only - ADVATE and diluent preassembled in - BAXJECT® III system - Do not open until time of use - Do not ...
-
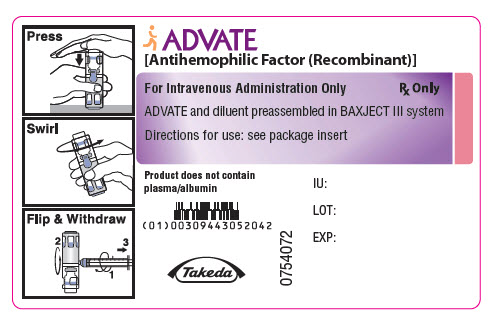
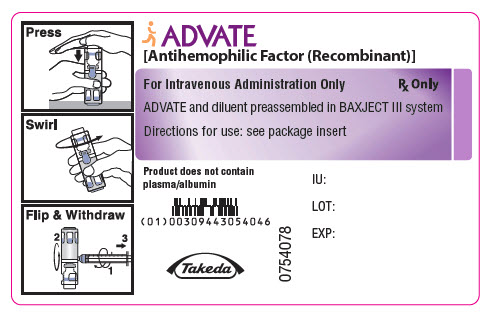
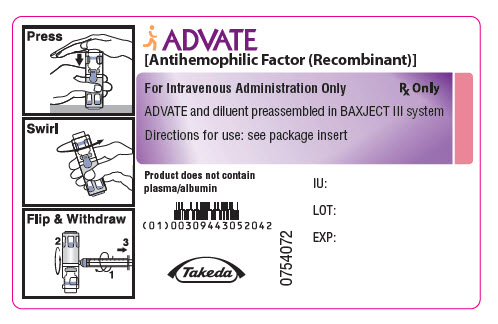
PRINCIPAL DISPLAY PANEL - 500 IU 2 mL Container LabelADVATE® [Antihemophilic Factor (Recombinant)] For Intravenous Administration Only - Rx Only - ADVATE and diluent preassembled in BAXJECT® III system - Directions for use: see package ...
-
PRINCIPAL DISPLAY PANEL - 1000 IU 2 mL Kit CartonADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3053-02 - Single-dose only - For Intravenous Administration Only - Rx Only - Lyophilized powder for reconstitution - ADVATE and diluent ...
-
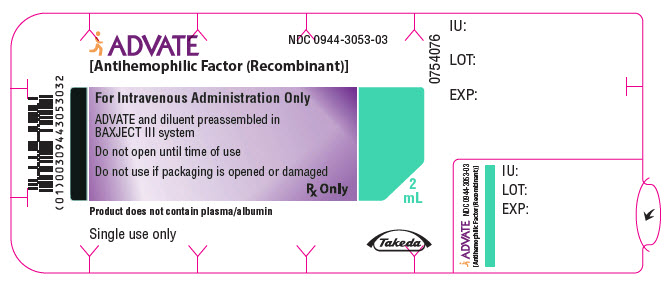
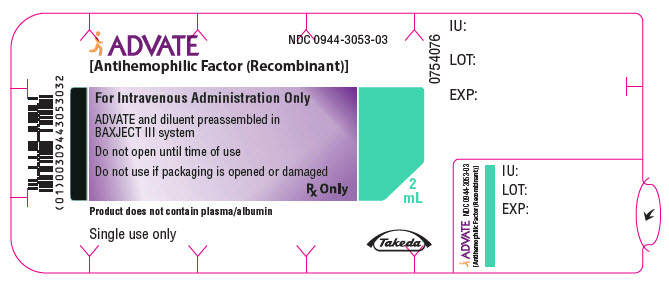
PRINCIPAL DISPLAY PANEL - 1000 IU 2 mL Blister Pack LabelADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3053-03 - For Intravenous Administration Only - ADVATE and diluent preassembled in - BAXJECT® III system - Do not open until time of use - Do not ...
-
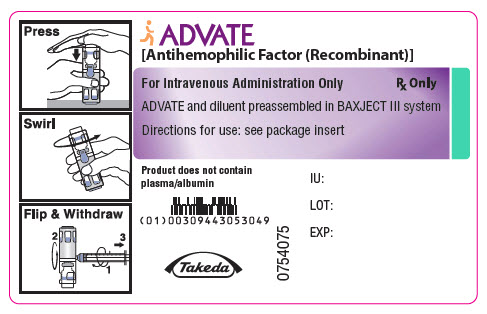
PRINCIPAL DISPLAY PANEL - 1000 IU 2 mL Container LabelADVATE® [Antihemophilic Factor (Recombinant)] For Intravenous Administration Only - Rx Only - ADVATE and diluent preassembled in BAXJECT® III system - Directions for use: see package ...
-
PRINCIPAL DISPLAY PANEL - 1500 IU 2 mL Kit CartonADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3054-02 - Single-dose only - For Intravenous Administration Only - Rx Only - Lyophilized powder for reconstitution - ADVATE and diluent ...
-
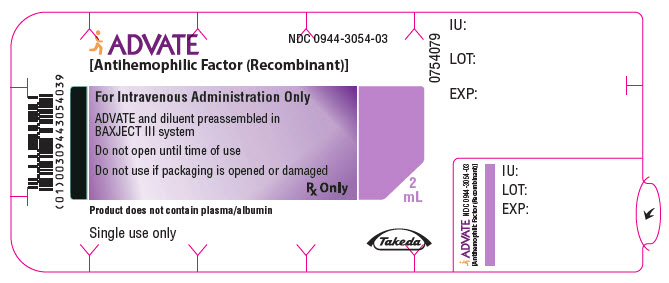
PRINCIPAL DISPLAY PANEL - 1500 IU 2 mL Blister Pack LabelADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3054-03 - For Intravenous Administration Only - ADVATE and diluent preassembled in - BAXJECT® III system - Do not open until time of use - Do not ...
-
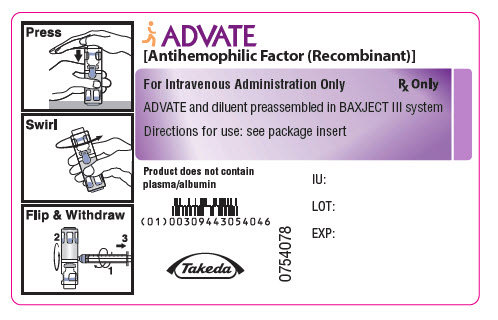
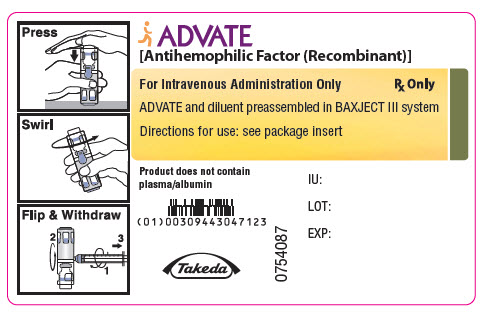
PRINCIPAL DISPLAY PANEL - 1500 IU 2 mL Container LabelADVATE® [Antihemophilic Factor (Recombinant)] For Intravenous Administration Only - Rx Only - ADVATE and diluent preassembled in BAXJECT® III system - Directions for use: see package ...
-
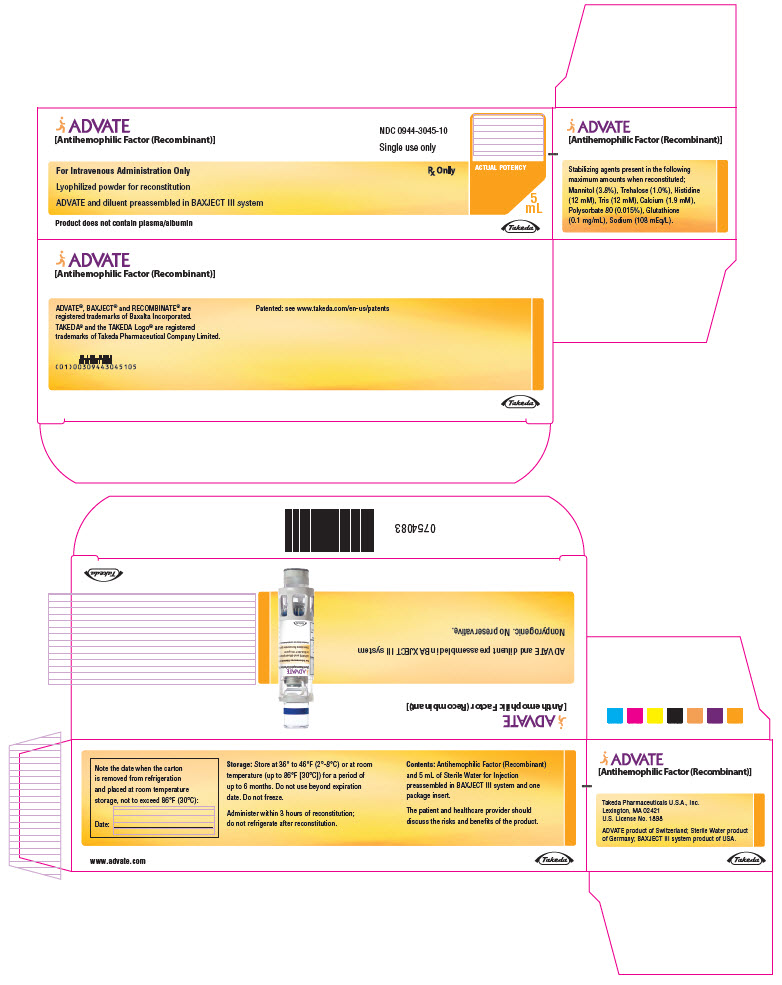
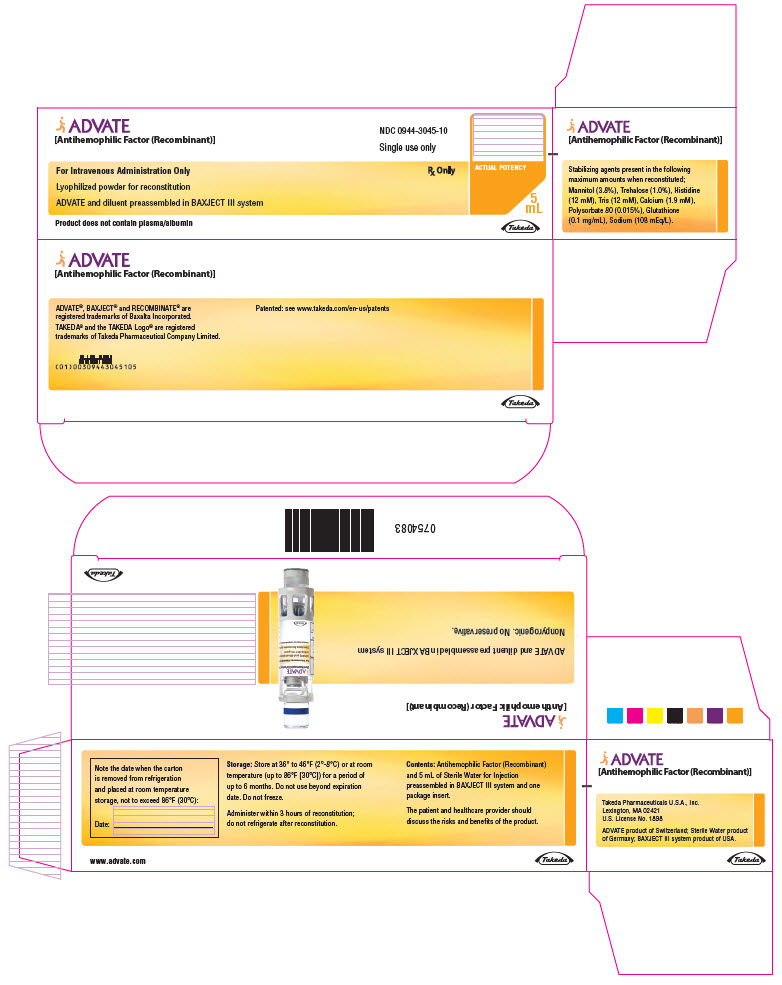
PRINCIPAL DISPLAY PANEL - 2000 IU 5 mL Kit CartonADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3045-10 - Single-dose only - For Intravenous Administration Only - Rx Only - Lyophilized powder for reconstitution - ADVATE and diluent ...
-
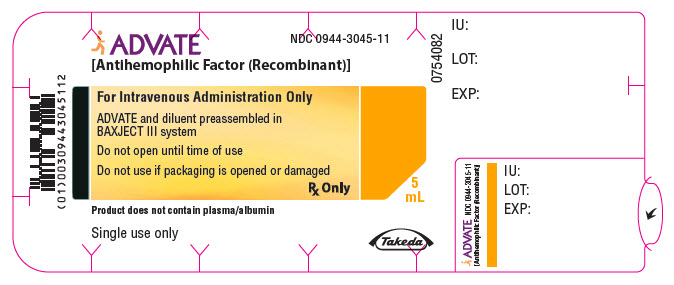
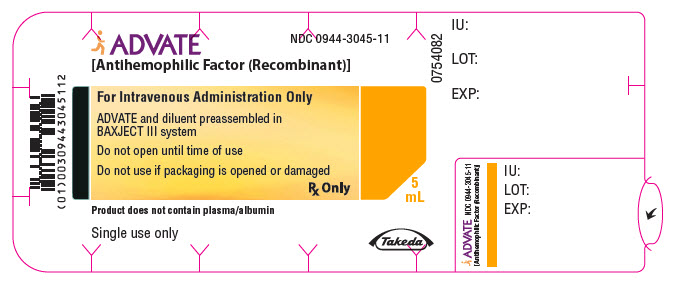
PRINCIPAL DISPLAY PANEL - 2000 IU 5 mL Blister Pack LabelADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3045-11 - For Intravenous Administration Only - ADVATE and diluent preassembled in - BAXJECT® III system - Do not open until time of use - Do not ...
-
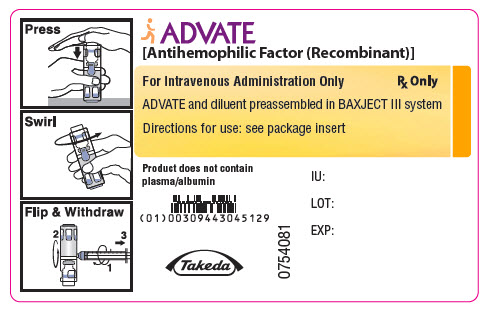
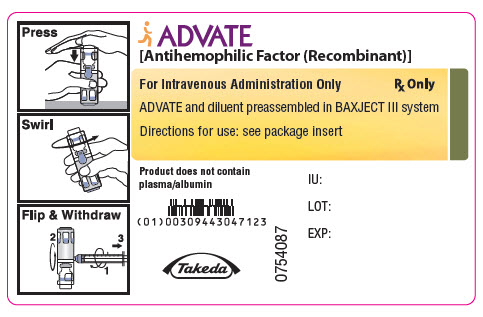
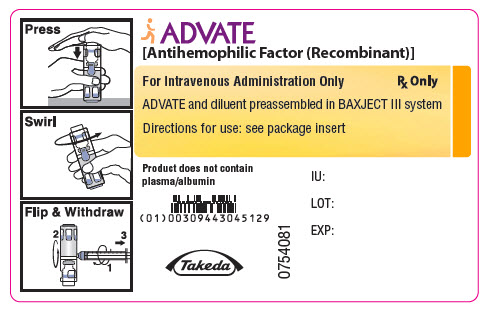
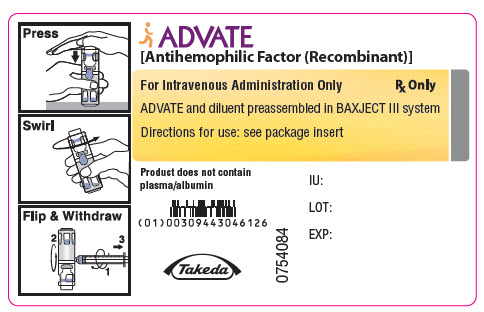
PRINCIPAL DISPLAY PANEL - 2000 IU 5 mL Container LabelADVATE® [Antihemophilic Factor (Recombinant)] For Intravenous Administration Only - Rx Only - ADVATE and diluent preassembled in BAXJECT® III system - Directions for use: see package ...
-
PRINCIPAL DISPLAY PANEL - 3000 IU 5 mL Kit CartonADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3046-10 - Single-dose only - For Intravenous Administration Only - Rx Only - Lyophilized powder for reconstitution - ADVATE and diluent ...
-
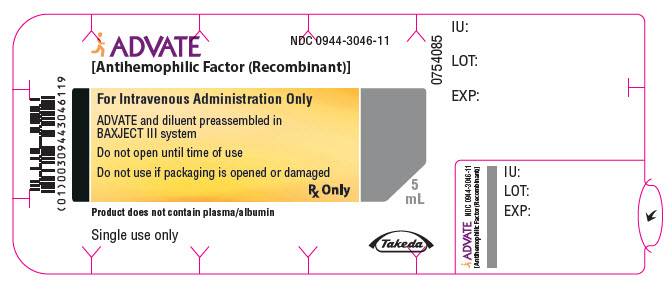
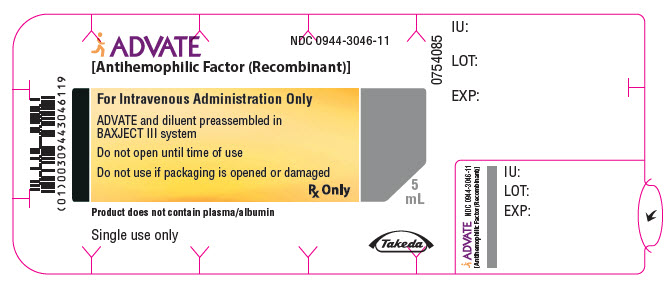
PRINCIPAL DISPLAY PANEL - 3000 IU 5 mL Blister Pack LabelADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3046-11 - For Intravenous Administration Only - ADVATE and diluent preassembled in - BAXJECT® III system - Do not open until time of use - Do not ...
-
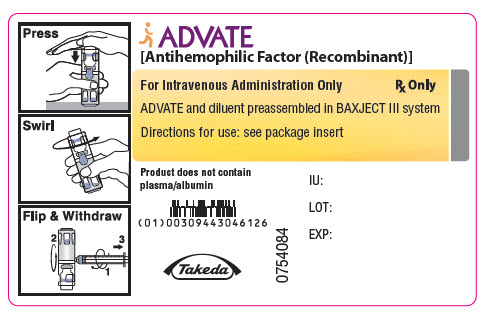
PRINCIPAL DISPLAY PANEL - 3000 IU 5 mL Container LabelADVATE® [Antihemophilic Factor (Recombinant)] For Intravenous Administration Only - Rx Only - ADVATE and diluent preassembled in BAXJECT® III system - Directions for use: see package ...
-
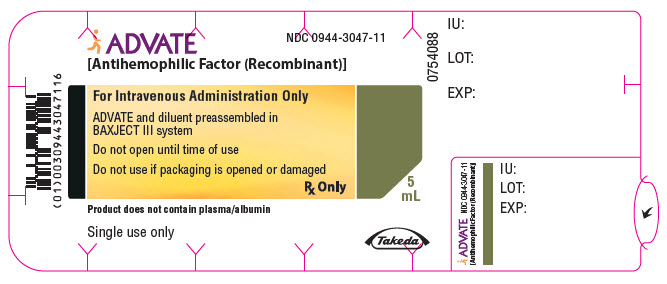
PRINCIPAL DISPLAY PANEL - 4000 IU 5 mL Kit CartonADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3047-10 - Single-dose only - For Intravenous Administration Only - Rx Only - Lyophilized powder for reconstitution - ADVATE and diluent ...
-
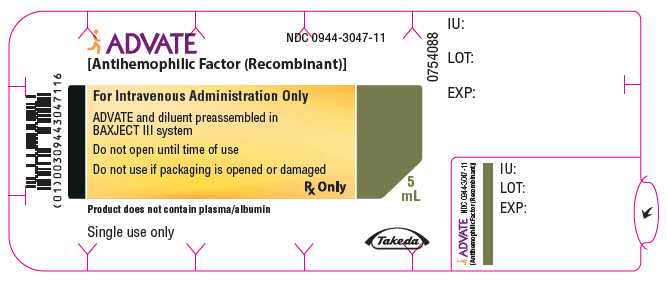
PRINCIPAL DISPLAY PANEL - 4000 IU 5 mL Blister Pack LabelADVATE® [Antihemophilic Factor (Recombinant)] NDC 0944-3047-11 - For Intravenous Administration Only - ADVATE and diluent preassembled in - BAXJECT® III system - Do not open until time of use - Do not ...
-
PRINCIPAL DISPLAY PANEL - 4000 IU 5 mL Container LabelADVATE® [Antihemophilic Factor (Recombinant)] For Intravenous Administration Only - Rx Only - ADVATE and diluent preassembled in BAXJECT® III system - Directions for use: see package ...
-
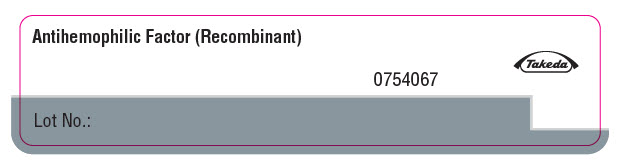
PRINCIPAL DISPLAY PANEL - Advate Vial LabelAntihemophilic Factor (Recombinant) Takeda - 0754067 - Lot No.:
-
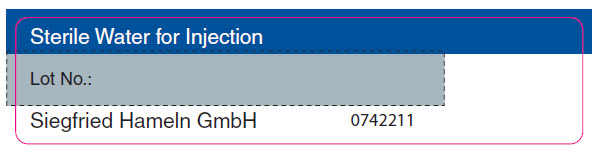
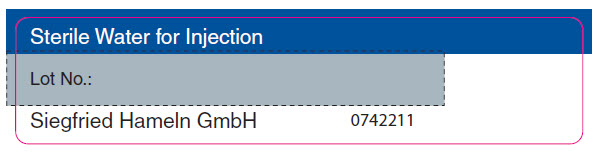
PRINCIPAL DISPLAY PANEL - Sterile Water for Injection Vial LabelSterile Water for Injection - Lot No.: Siegfried Hameln GmbH - 0742211
-
INGREDIENTS AND APPEARANCEProduct Information








 are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.