Label: TEPMETKO- tepotinib hydrochloride tablet
- NDC Code(s): 44087-5000-3, 44087-5000-6
- Packager: EMD Serono, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TEPMETKO safely and effectively. See full prescribing information for TEPMETKO. TEPMETKO® (tepotinib) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETEPMETKO is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations.
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection for METex14 Skipping Alterations - Select patients for treatment with TEPMETKO based on the presence of MET exon 14 skipping alterations in plasma or tumor specimens ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 225 mg, white-pink, oval, biconvex film-coated tablets with embossment "M" on one side and plain on the other side.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Interstitial Lung Disease (ILD)/Pneumonitis - ILD/pneumonitis, which can be fatal, occurred in patients treated with TEPMETKO [see Adverse Reactions (6.1)]. ILD/pneumonitis occurred in 2 ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in greater detail elsewhere in the labeling: Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.1)] Hepatotoxicity [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Effects of TEPMETKO on Other Drugs - Certain P-gp Substrates - Tepotinib is a P-gp inhibitor. Concomitant use of TEPMETKO increases the concentration of P-gp substrates [see Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animal studies and the mechanism of action [see Clinical Pharmacology (12.1)], TEPMETKO can cause fetal harm when administered to a pregnant ...
-
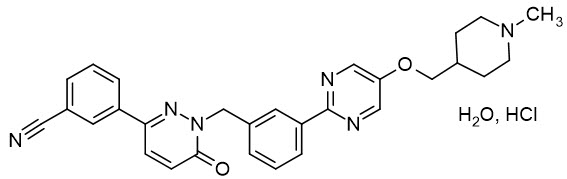
11 DESCRIPTIONTepotinib is a kinase inhibitor. TEPMETKO (tepotinib) tablets for oral use are formulated with tepotinib hydrochloride hydrate. The chemical name for tepotinib hydrochloride hydrate is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tepotinib is a kinase inhibitor that targets MET, including variants with exon 14 skipping alterations. Tepotinib inhibits hepatocyte growth factor (HGF)-dependent and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been performed with tepotinib. Tepotinib and its major circulating metabolite were not mutagenic in ...
-
14 CLINICAL STUDIESThe efficacy of TEPMETKO was evaluated in a single-arm, open-label, multicenter, non-randomized, multicohort study (VISION, NCT02864992). Eligible patients were required to have advanced or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTEPMETKO (tepotinib) tablets: 225 mg tepotinib, white-pink, oval, biconvex film-coated tablet with embossment "M" on one side and plain on the other side. NDC numberSize - 44087-5000-3Box ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Interstitial Lung Disease (ILD)/Pneumonitis - Inform patients of the risk of severe or fatal ILD/pneumonitis ...
-
SPL UNCLASSIFIED SECTIONManufactured for: EMD Serono, Inc. Boston, MA 02210 - U.S.A. TEPMETKO is a trademark of Merck KGaA, Darmstadt, Germany
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised 01/2025 - PATIENT INFORMATION - TEPMETKO® (tep-MET-co) (tepotinib) tablets, for oral use - What ...
-
PRINCIPAL DISPLAY PANEL - 30 Tablet Blister Pack CartonNDC 44087-5000-3 - TEPMETKO® (tepotinib) tablets - 225 mg per tablet - Rx Only - Each tablet contains 225 mg of tepotinib - (equivalent to 250 mg tepotinib hydrochloride hydrate) Each carton contains 3 ...
-
PRINCIPAL DISPLAY PANEL - 60 Tablet Blister Pack CartonNDC 44087-5000-6 - TEPMETKO® (tepotinib) tablets - 225 mg per tablet - Rx Only - Each tablet contains 225 mg of tepotinib - (equivalent to 250 mg tepotinib hydrochloride hydrate) Each carton contains 6 ...
-
INGREDIENTS AND APPEARANCEProduct Information