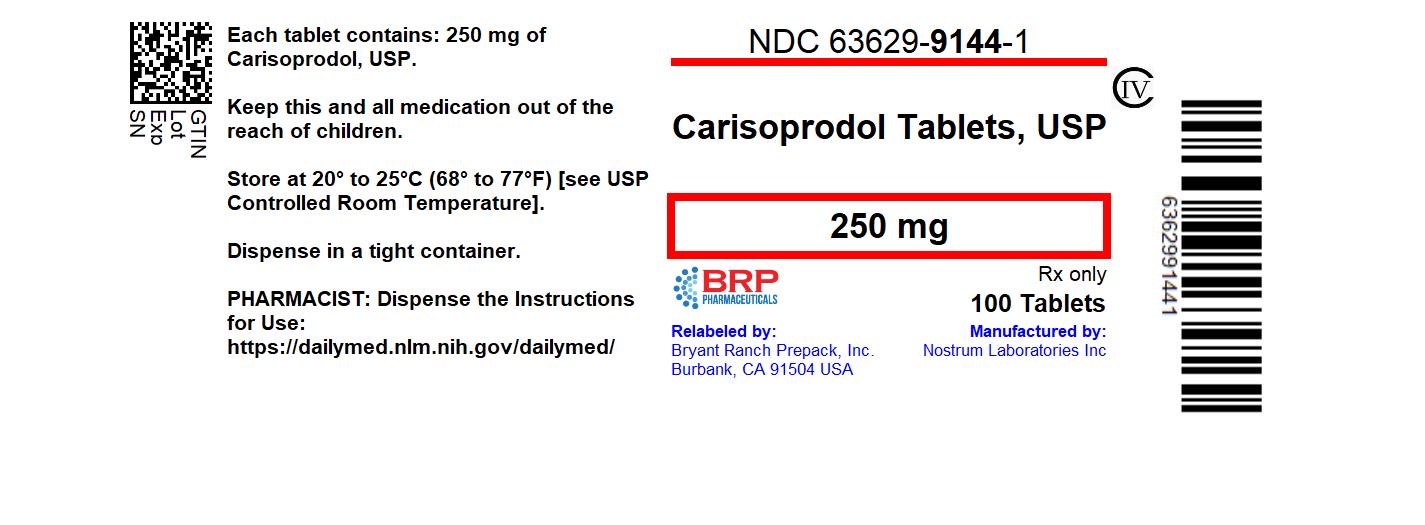
Label: CARISOPRODOL- carisoprodol tablet
- NDC Code(s): 63629-9144-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 29033-207
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CARISOPRODOL TABLETS safely and effectively. See full prescribing information for CARISOPRODOL TABLETS. CARISOPRODOL tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECarisoprodol tablets are indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults. Limitation of Use - Carisoprodol tablets should only be used ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose of carisoprodol tablet is 250 mg to 350 mg three times a day and at bedtime. The recommended maximum duration of carisoprodol tablets use is up to two or three weeks.
-
3 DOSAGE FORMS AND STRENGTHS250 mg Tablets: Round,white to off-white tablets, debossed with "NC" on one side and "250" on other side. 350 mg Tablets: Round, white to off white tablets, debossed with “NC” on one side and ...
-
4 CONTRAINDICATIONSCarisoprodol tablets are contraindicated in patients with a history of acute intermittent porphyria or a hypersensitivity reaction to a carbamate such as meprobamate.
-
5 WARNINGS AND PRECAUTIONS5.1 Sedation - Carisoprodol tablets have sedative properties (in the low back pain trials, 13% to 17% of patients who received carisoprodol tablets experienced sedation compared to 6% of patients ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared ...
-
7 DRUG INTERACTIONS7.1 CNS Depressants - The sedative effects of carisoprodol tablets and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive. Therefore ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Data over many decades of carisoprodol use in pregnancy have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Carisoprodol tablets contain carisoprodol, a Schedule IV controlled substance. Carisoprodol has been subject to abuse, misuse, and criminal diversion for nontherapeutic ...
-
10 OVERDOSAGEClinical Presentation - Overdosage of carisoprodol tablets commonly produces CNS depression. Death, coma, respiratory depression, hypotension, seizures, delirium, hallucinations, dystonic ...
-
11 DESCRIPTIONCarisoprodol tablets, USP are available as 250 mg and 350 mg round, white to off-white tablets. Carisoprodol is a white, crystalline powder, having a mild, characteristic odor and a bitter taste ...
-
12 CLINCIAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of carisoprodol in relieving discomfort associated with acute painful musculoskeletal conditions has not been clearly identified. In animal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinigenesis - Long term studies in animals have not been performed to evaluate the carcinogenic potential of ...
-
14 CLINICAL STUDIESThe safety and efficacy of carisoprodol tablets for the relief of acute, idiopathic mechanical low back pain was evaluated in two, 7-day, double blind, randomized, multicenter, placebo controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING250 mg Tablets: Round, white to off-tablets, debossed with "NC" on one side and "250" on other side; available in bottles of 100 (NDC 63629-9144-1). Storage: Store at controlled room temperature ...
-
17 PATIENT COUNSELING INFORMATIONPatients should be advised to contact their physician if they experience any adverse reactions to carisoprodol tablets. Sedation - Advise patients that carisoprodol tablets may cause drowsiness ...
-
PRINCIPAL DISPLAY PANELCarisoprodol 250 mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information