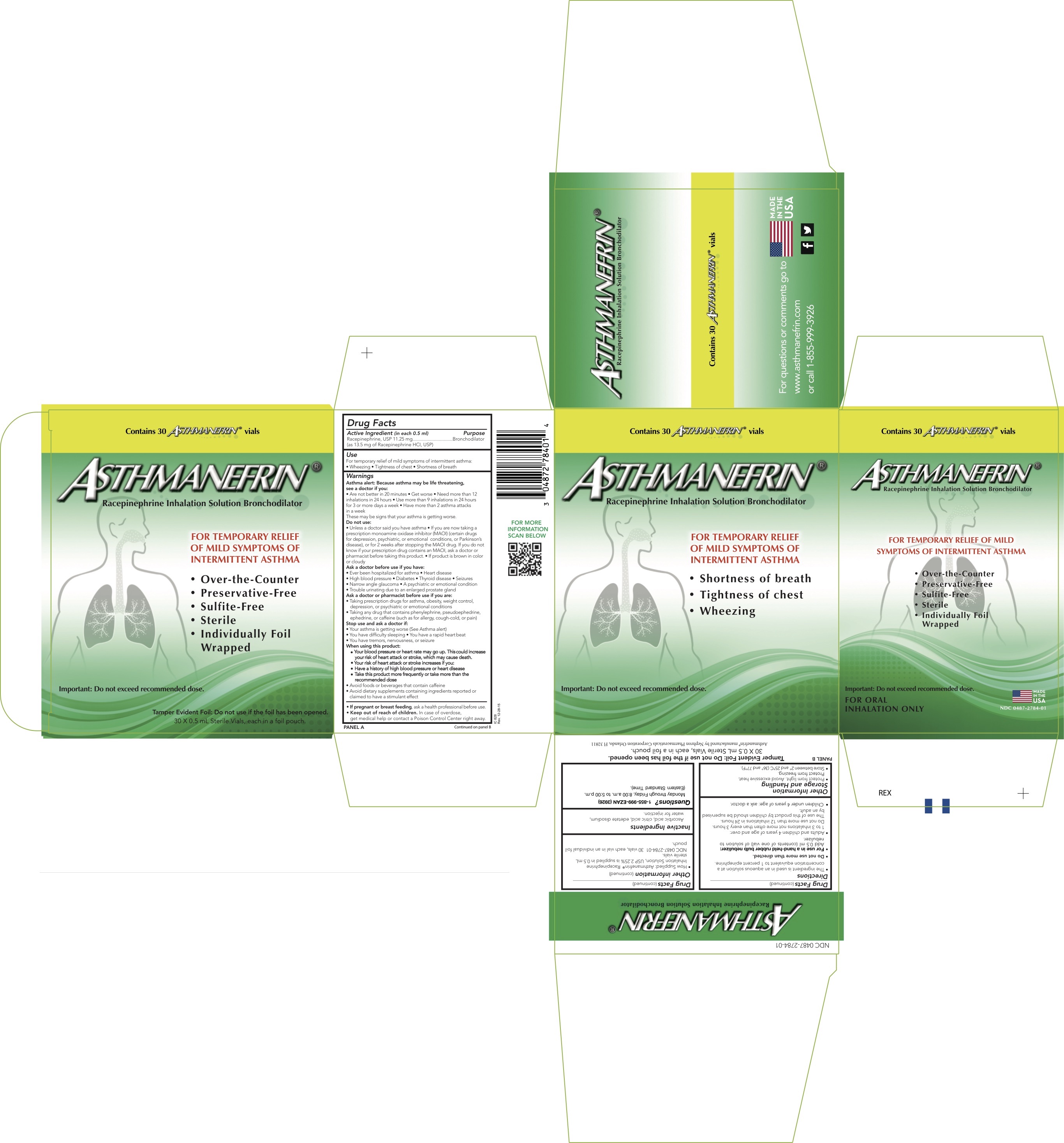
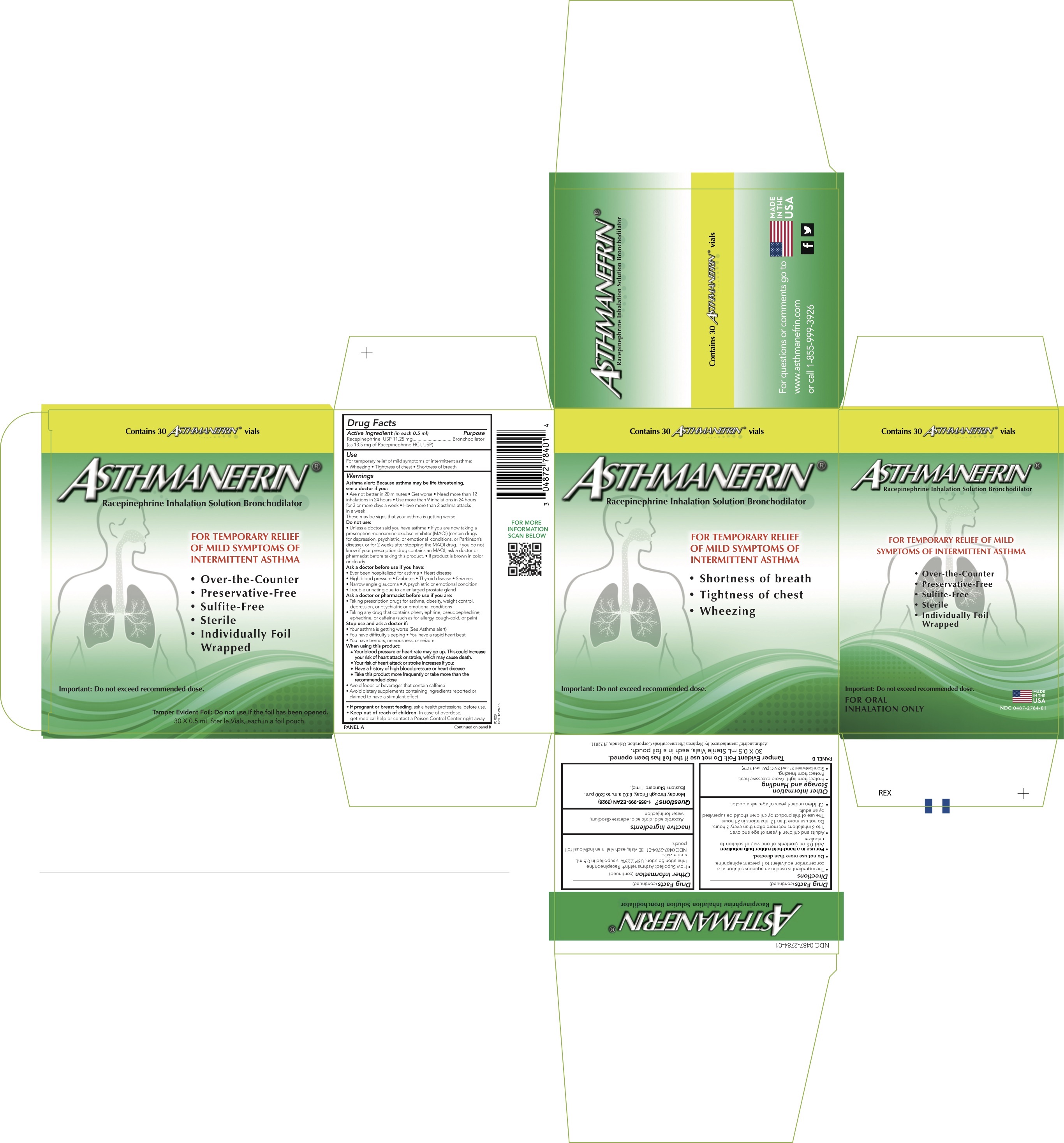
Label: ASTHMANEFRIN- racepinephrine hydrochloride solution
- NDC Code(s): 0487-2784-01
- Packager: Nephron Pharmaceuticals Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 8, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each 0.5 ml)Racepinephrine, USP 11.25 mg (as 13.5 mg of Racepinephrine HCl, USP).
-
PurposeBronchodilator
-
UsesFor temporary relief of mild symptoms of intermittent asthma: Wheezing - Tightness of chest - Shortness of breath
-
WarningsAsthma alert: Because asthma may be life threatening, see a doctor if you: Are not better in 20 minutes - Get worse - Need more than 12 inhalations in 24 hours - Use more than 9 inhalations in 24 ...
-
DirectionsThe ingredient is used in an aqueous solution at a concentration equivalent to 1 percent epinephrine. Do not use more than directed - For use in a hand-held rubber bulb nebulizer: Add 0.5 mL ...
-
Other InformationStorage and Handling - Protect from light. Avoid excessive heat. Protect from freezing. Store between 2°C and 25°C (36°F and 77°F). How Supplied: Asthmanefrin - ® Racepinephrine ...
-
Inactive IngredientsAscorbic acid, citric acid, edetate disodium, water for injection.
-
Questions?1-855-999-EZAN (3926) Monday through Friday, 8:00 a.m. to 5:00 p.m. (Eastern Standard Time).
-
SPL UNCLASSIFIED SECTIONManufactured By: nephron - pharmaceuticals - corporation - West Columbia, SC 29172
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial CartonContains 30 - ASTHMANEFRIN® vials - ASTHMANEFRIN - ® Racepinephrine Inhalation Solution Bronchodilator - FOR TEMPORARY RELIEF - OF MILD SYMPTOMS OF - INTERMITTENT ...
-
INGREDIENTS AND APPEARANCEProduct Information