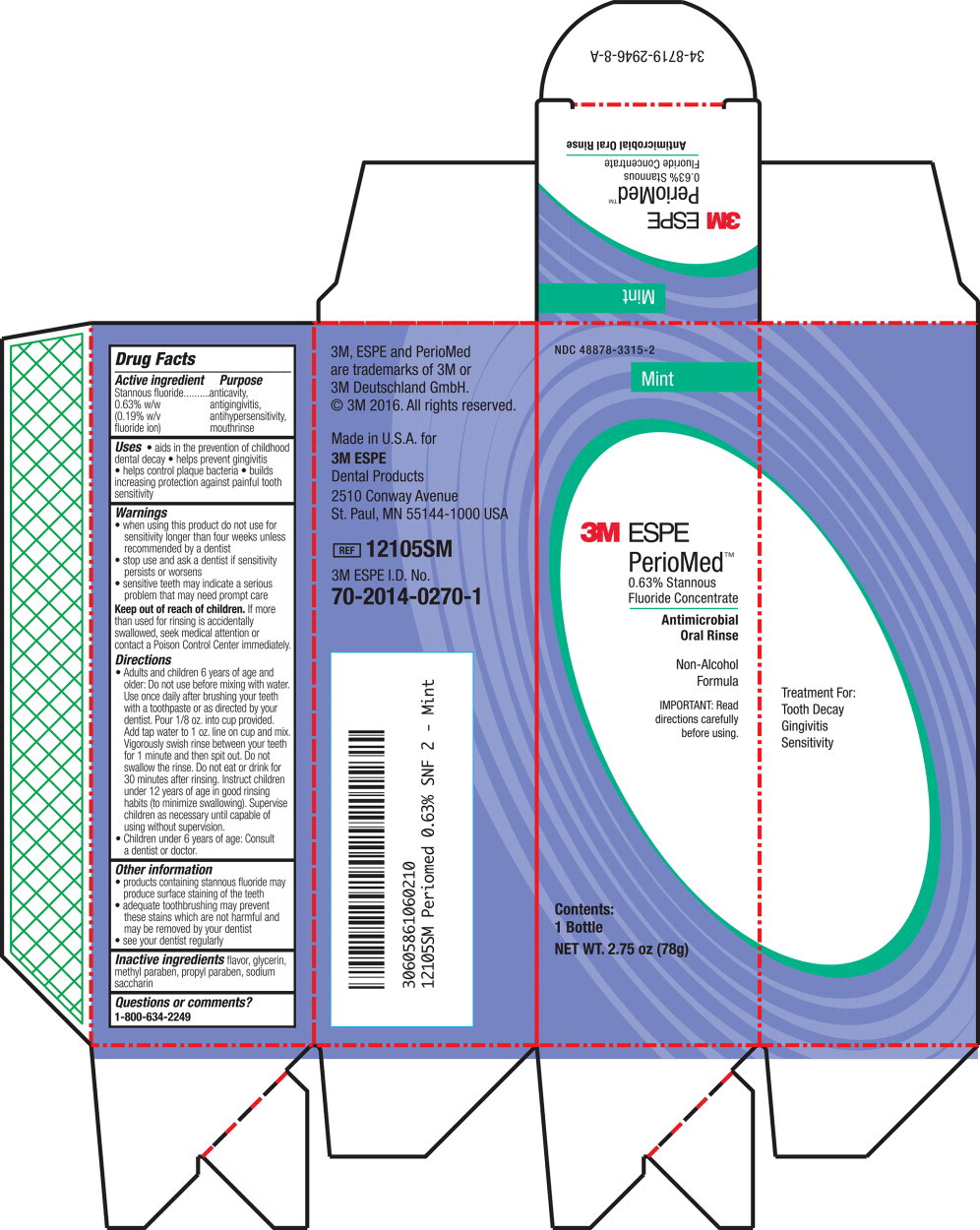
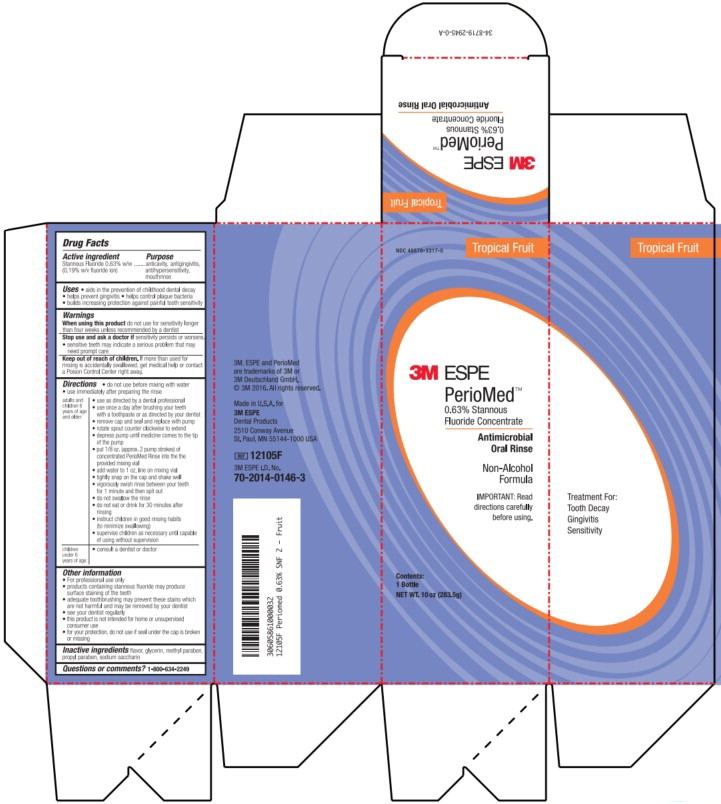
Label: PERIOMED- stannous fluoride rinse
- NDC Code(s): 48878-3315-0, 48878-3315-2, 48878-3317-0
- Packager: Solventum US OpCo LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
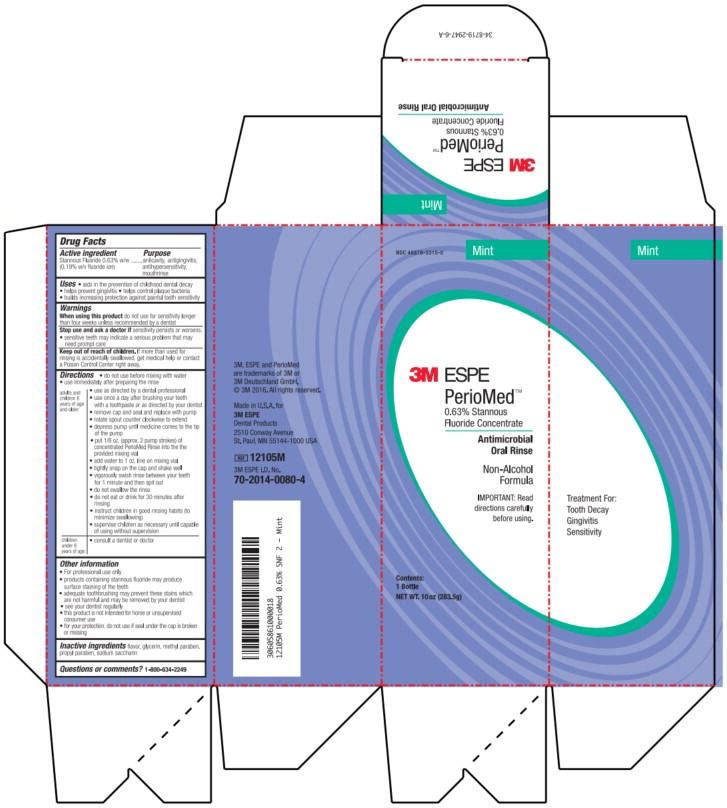
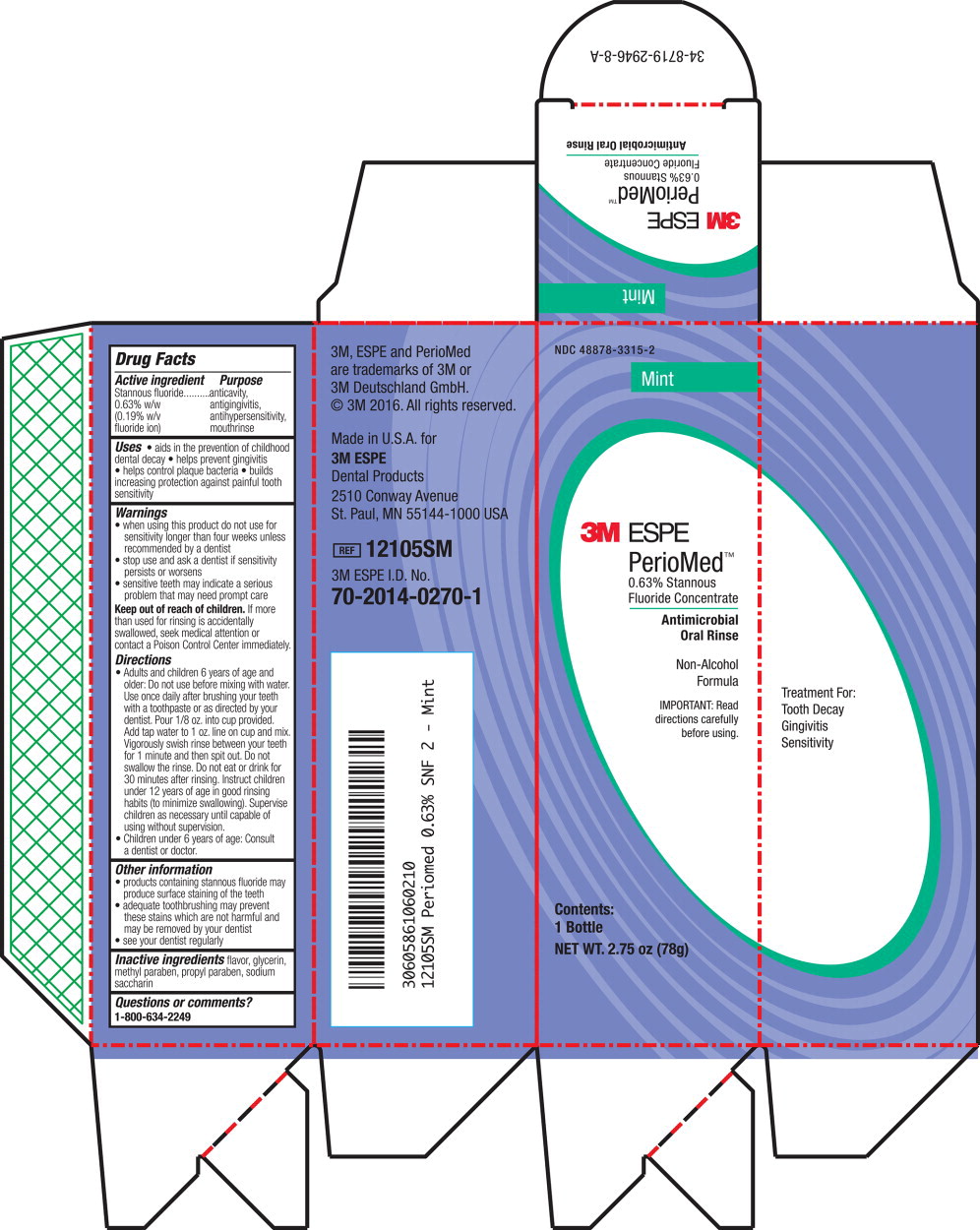
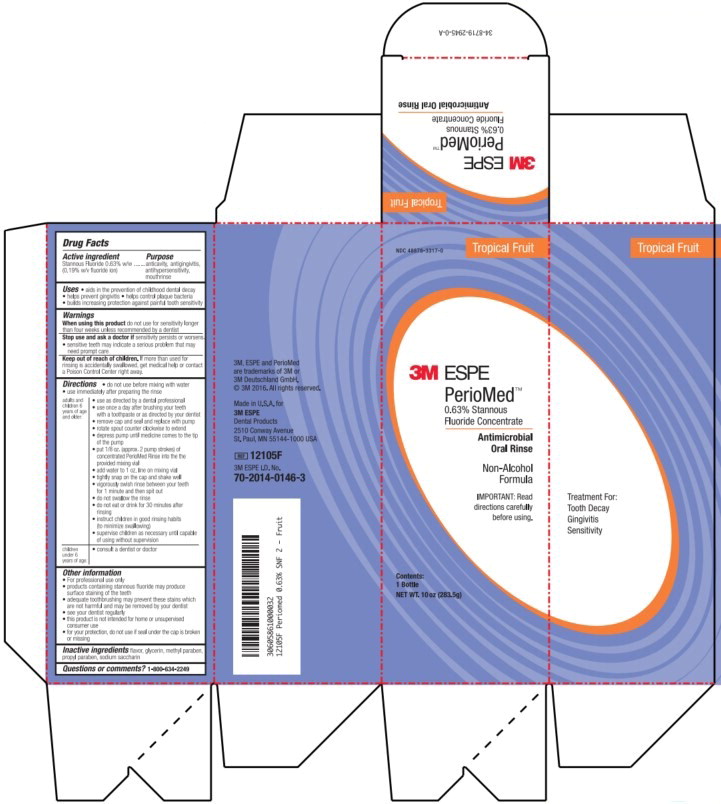
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
adults and children 6 years of age and older:
- do not use before mixing with water
- use immediately after preparing the rinse
- use as directed by a dental professional
- use once a day after brushing your teeth with a toothpaste or as directed by your dentist
- remove cap and seal and replace with pump
- rotate spout counter clockwise to extend
- depress pump until medicine comes to the tip of the pump
- put 1/8 oz. (approx. 2 pump strokes) of concentrated PerioMed Rinse into the provided mixing vial
- add water to 1 oz. line on mixing vial
- tightly snap on the cap and shake well
- vigorously swish rinse between your teeth for 1 minute and then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
children under 6 years of age:
- consult a dentist or doctor
-
Other information
- For professional use only
- products containing stannous fluoride may produce surface staining of the teeth
- adequate toothbrushing may prevent these stains which are not harmful and may be removed by your dentist
- see your dentist regularly
- this product is not intended for home or unsupervised consumer use
- for your protection, do not use if seal under the cap is broken or missing
- Inactive ingredients
- Questions or comments?
- Principal Display Panel – Box Label (Mint – 283.5 g)
- Principal Display Panel – Box Label (Mint – 78 g)
- Principal Display Panel – Box Label (Tropical Fruit – 283.5 g)
-
INGREDIENTS AND APPEARANCE
PERIOMED
stannous fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:48878-3315 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Stannous Fluoride (UNII: 3FTR44B32Q) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 1.53 mg in 1 g Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Saccharin (UNII: FST467XS7D) Product Characteristics Color Score Shape Size Flavor MINT (MINT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48878-3315-0 1 in 1 BOX 07/01/1967 1 283.5 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:48878-3315-2 1 in 1 BOX 02/01/2016 2 85 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 07/01/1967 PERIOMED
stannous fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:48878-3317 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Stannous Fluoride (UNII: 3FTR44B32Q) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 1.53 mg in 1 g Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Saccharin (UNII: FST467XS7D) Product Characteristics Color Score Shape Size Flavor FRUIT (FRUIT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48878-3317-0 1 in 1 BOX 07/01/1967 1 283.5 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 07/01/1967 Labeler - Solventum US OpCo LLC (801390852)