Label: XOLREMDI- mavorixafor capsule, gelatin coated
- NDC Code(s): 83296-100-12, 83296-100-60, 83296-100-90
- Packager: X4 Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use XOLREMDI™ safely and effectively. See full prescribing information for XOLREMDI - ®. XOLREMDI - ®(mavorixafor) capsules, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEXOLREMDI is indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of XOLREMDI is: Weight more than 50 kg: 400 mg orally once daily on an empty stomach after an overnight fast, and at least 30 minutes before ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 100 mg, opaque hard gelatin capsules with white body and light blue cap. The white capsule body is imprinted with "100 mg" in black ink, and the light blue capsule cap is imprinted ...
-
4 CONTRAINDICATIONSUse of XOLREMDI is contraindicated with drugs that are highly dependent on CYP2D6 for clearance - [see - Drug Interactions (7.2)] .
-
5 WARNINGS AND PRECAUTIONS5.1 Embryo-Fetal Toxicity - Based on its mechanism of action, XOLREMDI is expected to cause fetal harm when administered to a pregnant woman - [see - Clinical Pharmacology (12.2)] ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: QTc Interval Prolongation - [see - Warnings and Precautions (5.2)] 6.1 ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on XOLREMDI - Strong or Moderate CYP3A4 Inhibitors - Reduce XOLREMDI daily dosage to 200 mg when used concomitantly with a strong CYP3A4 inhibitor - [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, XOLREMDI is expected to cause fetal harm when administered to a pregnant woman - [see - Clinical Pharmacology (12.1) ...
-
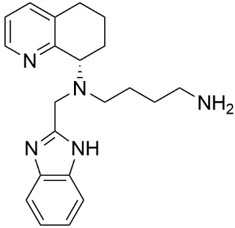
11 DESCRIPTIONMavorixafor is an orally bioavailable CXC Chemokine Receptor 4 (CXCR4) antagonist - [see - Clinical Pharmacology (12.1)] . The chemical name of the active ingredient, mavorixafor, is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mavorixafor is an orally bioavailable CXCR4 antagonist that blocks the binding of the CXCR4 ligand, stromal-derived factor-1α (SDF-1α)/CXC Chemokine Ligand 12 (CXCL12) ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies with mavorixafor have not been conducted. Mavorixafor was not genotoxic in an - in vitrobacterial reverse ...
-
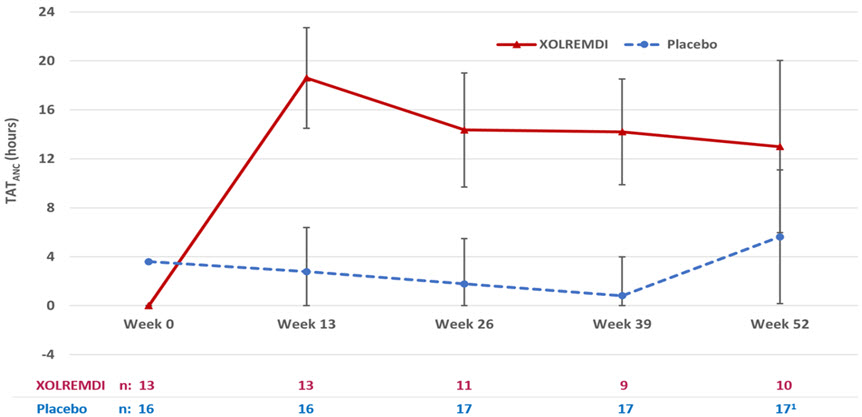
14 CLINICAL STUDIESThe efficacy of XOLREMDI in patients aged 12 and older with WHIM syndrome was demonstrated in the 52-week, randomized, double-blind, placebo-controlled portion of Study 1 [ NCT03995108] ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGXOLREMDI is supplied as an opaque white, hard gelatin capsule with a light blue cap, containing 100 mg of the active ingredient mavorixafor. The white capsule body is axially imprinted with "100 ...
-
17 PATIENT COUNSELING INFORMATIONAdministration - Advise patients to take XOLREMDI on an empty stomach after an overnight fast, 30 minutes before food. Advise patients to swallow the capsules whole and not to open, break, or ...
-
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle LabelNDC 83296-100-60 - XOLREMDI™ (mavorixafor) capsules - 100 mg - Swallow the capsules whole. Do not open, break, or chew capsules. Rx only - 60 capsules
-
INGREDIENTS AND APPEARANCEProduct Information