Label: PHENAZOPYRIDINE HYDROCHLORIDE tablet
- NDC Code(s): 70518-0218-0, 70518-0218-1, 70518-0218-2
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 75826-114
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
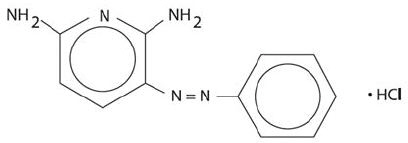
DESCRIPTIONPhenazopyridine Hydrochloride is dark brown fine granular powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain. Phenazopyridine HCl tablets ...
-
CLINICAL PHARMACOLOGYPhenazopyridine HCl is excreted in the urine where it exerts a topical analgesic effect on the mucosa of the urinary tract. This action helps to relieve pain, burning, urgency and frequency ...
-
INDICATIONS AND USAGEPhenazopyridine HCl is indicated for the symptomatic relief of pain, burning, urgency, frequency, and other discomforts arising from irritation of the lower urinary tract mucosa caused by ...
-
CONTRAINDICATIONSPhenazopyridine HCl should not be used in patients who have previously exhibited hypersensitivity to it. The use of Phenazopyridine HCl is contraindicated in patients with ...
-
ADVERSE REACTIONSHeadache, rash, pruritus and occasional gastrointestinal disturbance. An anaphylactoid-like reaction has been described. Methemoglobinemia, hemolytic anemia, renal and hepatic toxicity have been ...
-
PRECAUTIONSGeneral:A yellowish tinge of the skin or sclera may indicate accumulation due to impaired renal excretion and the need to discontinue therapy. The decline in renal function associated with ...
-
DOSAGE AND ADMINISTRATION100 mg Tablets: Average adult dosage is two tablets 3 times a day after meals. When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the ...
-
OVERDOSAGEExceeding the recommended dose in patients with good renal function or administering the usual dose to patients with impaired renal function (common in elderly patients) may lead to increased ...
-
HOW SUPPLIED100 mg Tablets: Appearance: Dark brown to maroon colored, round, film coated tablets debossed "114" on one side and plain on the other side. NDC: 70518-0218-00 - NDC: 70518-0218-01 - NDC ...
-
PRINCIPAL DISPLAY PANELDRUG: Phenazopyridine Hydrochloride - GENERIC: Phenazopyridine Hydrochloride - DOSAGE: TABLET - ADMINSTRATION: ORAL - NDC: 70518-0218-0 - NDC: 70518-0218-1 - NDC: 70518-0218-2 - COLOR: brown - SHAPE: ROUND - SCORE ...
-
INGREDIENTS AND APPEARANCEProduct Information