Label: SUCRALFATE- sucralfate oral suspension
- NDC Code(s): 52817-840-16
- Packager: TruPharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
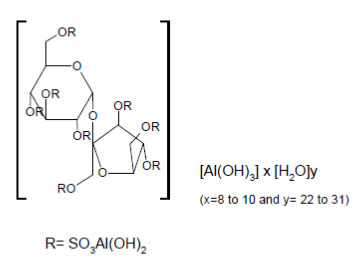
DESCRIPTIONSucralfate oral suspension contains sucralfate and sucralfate is an α-D-glucopyranoside, β-D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. Sucralfate oral suspension for oral ...
-
CLINICAL PHARMACOLOGYSucralfate is only minimally absorbed from the gastrointestinal tract. The small amounts of the sulfated disaccharide that are absorbed are excreted primarily in the urine. Although the mechanism ...
-
CLINICAL TRIALSIn a multicenter, double-blind, placebo-controlled study of sucralfate oral suspension, a dosage regimen of 1 gram (10 mL) four times daily was demonstrated to be superior to placebo in ulcer ...
-
INDICATIONS AND USAGESucralfate Oral Suspension is Sucralfate oral suspension is indicated in the short-term (up to 8 weeks) treatment of active duodenal ulcer.in the short-term (up to 8 weeks) treatment of active ...
-
CONTRAINDICATIONSSucralfate oral suspension is contraindicated for patients with known hypersensitivity reactions to the active substance or to any of the excipients.
-
WARNINGS
Fatal complications, including pulmonary and cerebral emboli have occurred with inappropriate intravenous administration of sucralfate oral suspension. Administer sucralfate oral suspension only ...
-
PRECAUTIONSThe physician should read the "PRECAUTIONS" section when considering the use of sucralfate oral suspension in pregnant or pediatric patients, or patients of childbearing potential. Duodenal ulcer ...
-
ADVERSE REACTIONSAdverse reactions to sucralfate tablets in clinical trials were minor and only rarely led to discontinuation of the drug. In studies involving over 2,700 patients treated with sucralfate, adverse ...
-
OVERDOSAGEDue to limited experience in humans with overdosage of sucralfate, no specific treatment recommendations can be given. Acute oral studies in animals, however, using doses up to 12 g/kg body ...
-
DOSAGE AND ADMINISTRATIONActive Duodenal Ulcer: The recommended adult oral dosage for duodenal ulcer is 1 gram (10 mL) four times per day. Sucralfate oral suspension should be administered on an empty stomach. Antacids ...
-
HOW SUPPLIEDSucralfate oral suspension 1 g/10 mL is a pink suspension supplied in the following dosage form: 16 fl oz (473 mL) Bottle. NDC # 52817-840-16. SHAKE WELL BEFORE USING. AVOID ...
-
PRINCIPAL DISPLAY PANELNDC 52817-840-16 - Sucralfate - Oral Suspension - 1 g/10 mL - FOR ORAL ADMINSTRATION ONLY - Each 10 mL (2 teaspoons) Sucralfate Oral Suspension contains 1 g sucralfate. SHAKE ...
-
INGREDIENTS AND APPEARANCEProduct Information