Label: ROFLUMILAST tablet
- NDC Code(s): 59651-275-30, 59651-275-90, 59651-801-20, 59651-801-28
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ROFLUMILAST TABLETS safely and effectively. See full prescribing information for ROFLUMILAST TABLETS. ROFLUMILAST tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERoflumilast tablets are indicated as a treatment to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of ...
-
2 DOSAGE AND ADMINISTRATIONThe maintenance dose of roflumilast tablet is one 500 micrograms (mcg) tablet per day, with or without food. Starting treatment with a dose of roflumilast tablets 250 mcg once daily for 4 weeks ...
-
3 DOSAGE FORMS AND STRENGTHSRoflumilast 250 mcg tablets are white to off-white, round tablets, flat faced beveled edge, debossed with “T” and “250” on one side and plain on the other side. Roflumilast 500 mcg tablets are ...
-
4 CONTRAINDICATIONSThe use of roflumilast tablets are contraindicated in the following condition: Moderate to severe liver impairment (Child-Pugh B or C) [see Clinical Pharmacology (12.3) and Use in Specific ...
-
5 WARNINGS AND PRECAUTIONS5.1 Treatment of Acute Bronchospasm - Roflumilast is not a bronchodilator and should not be used for the relief of acute bronchospasm. 5.2 Psychiatric Events including Suicidality - Treatment ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in greater detail in other sections: Psychiatric Events Including Suicidality [see Warnings and Precautions (5.2)] Weight Decrease [see Warnings ...
-
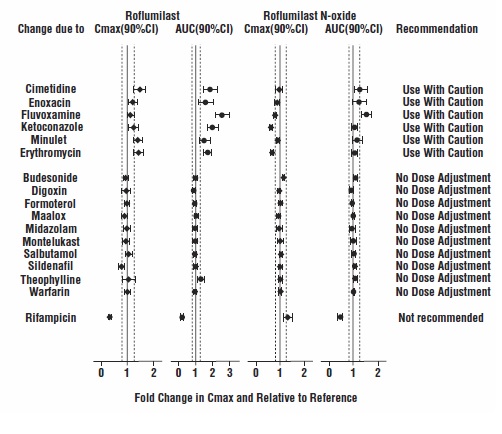
7 DRUG INTERACTIONSA major step in roflumilast metabolism is the N-oxidation of roflumilast to roflumilast N-oxide by CYP3A4 and CYP1A2 [see Clinical Pharmacology (12.3)]. 7.1 Drugs that Induce Cytochrome P450 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no randomized clinical studies of roflumilast in pregnant women. In animal reproductive toxicity studies, roflumilast administered to pregnant rats and ...
-
10 OVERDOSAGE10.1 Human Experience - No case of overdose has been reported in clinical studies with roflumilast. During the Phase I studies of roflumilast, the following symptoms were observed at an increased ...
-
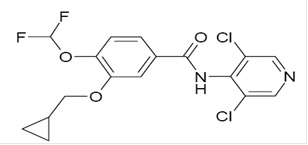
11 DESCRIPTIONThe active ingredient in roflumilast tablets is roflumilast, USP. Roflumilast and its active metabolite (roflumilast N-oxide) are selective phosphodiesterase 4 (PDE4) inhibitors. The chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Roflumilast and its active metabolite (roflumilast N-oxide) are selective inhibitors of phosphodiesterase 4 (PDE4). Roflumilast and roflumilast N-oxide inhibition of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies were conducted in hamsters and mice with roflumilast to evaluate its carcinogenic potential. In 2-year oral gavage ...
-
14 CLINICAL STUDIES14.1 Chronic Obstructive Pulmonary Disease (COPD) The efficacy and safety of roflumilast in COPD was evaluated in 8 randomized, double-blind, controlled, parallel-group clinical trials in 9394 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Roflumilast tablets, 250 mcg are supplied as white to off-white, round tablets, flat faced beveled edge, debossed with “T” and “250” on one side and plain on the other ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Bronchospasm - Roflumilast is not a bronchodilator and should not be used for immediate relief of breathing ...
-
MEDICATION GUIDERoflumilast Tablets - (roe flue' mi last) Read this Medication Guide before you start taking roflumilast tablets and each time you get a refill. There may be new information. This information ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mcg (28 Tablets Wallet Carton)NDC 59651-801-28 - Rx only - Roflumilast Tablets - 250 mcg - PHARMACIST: Dispense the Medication Guide - provided separately to each patient. 28 Day Pack - CONTENTS: One Child-Resistant Package ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mcg (28 Tablets Blister Wallet)NDC 59651-801-28 - Rx only - Roflumilast Tablets - 250 mcg - PHARMACIST: Dispense the Medication Guide - provided separately to each patient. 28 Day Pack - AUROBINDO 28 ...
-
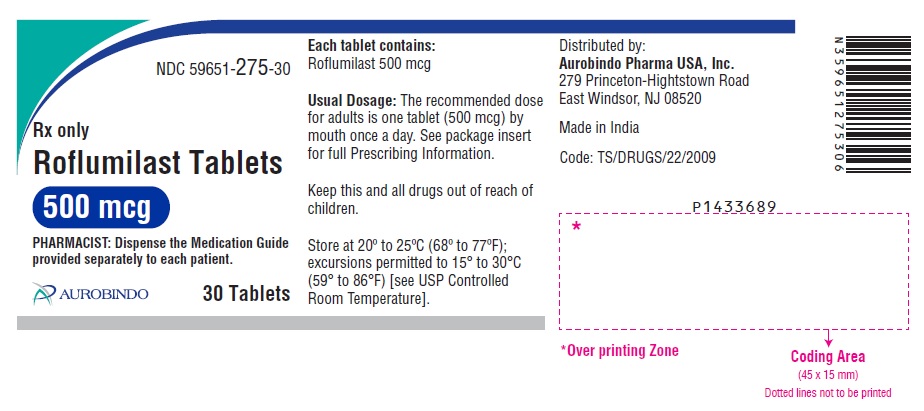
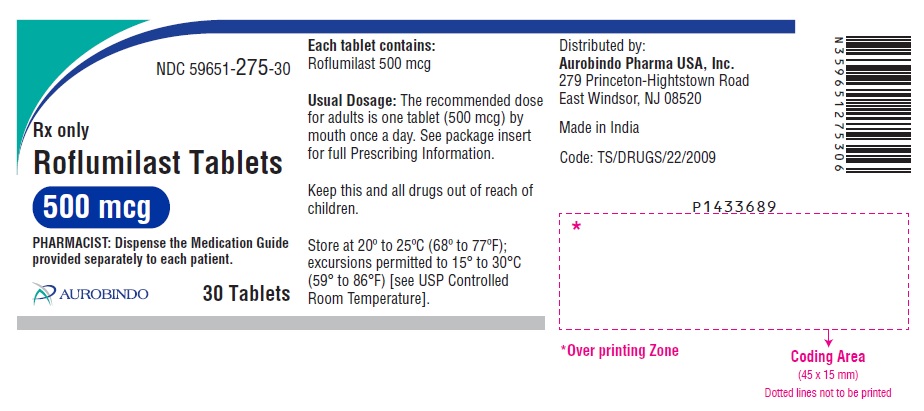
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mcg (30 Tablet Bottle)NDC 59651-275-30 - Rx only - Roflumilast Tablets - 500 mcg - PHARMACIST: Dispense the Medication Guide - provided separately to each patient. AUROBINDO 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information