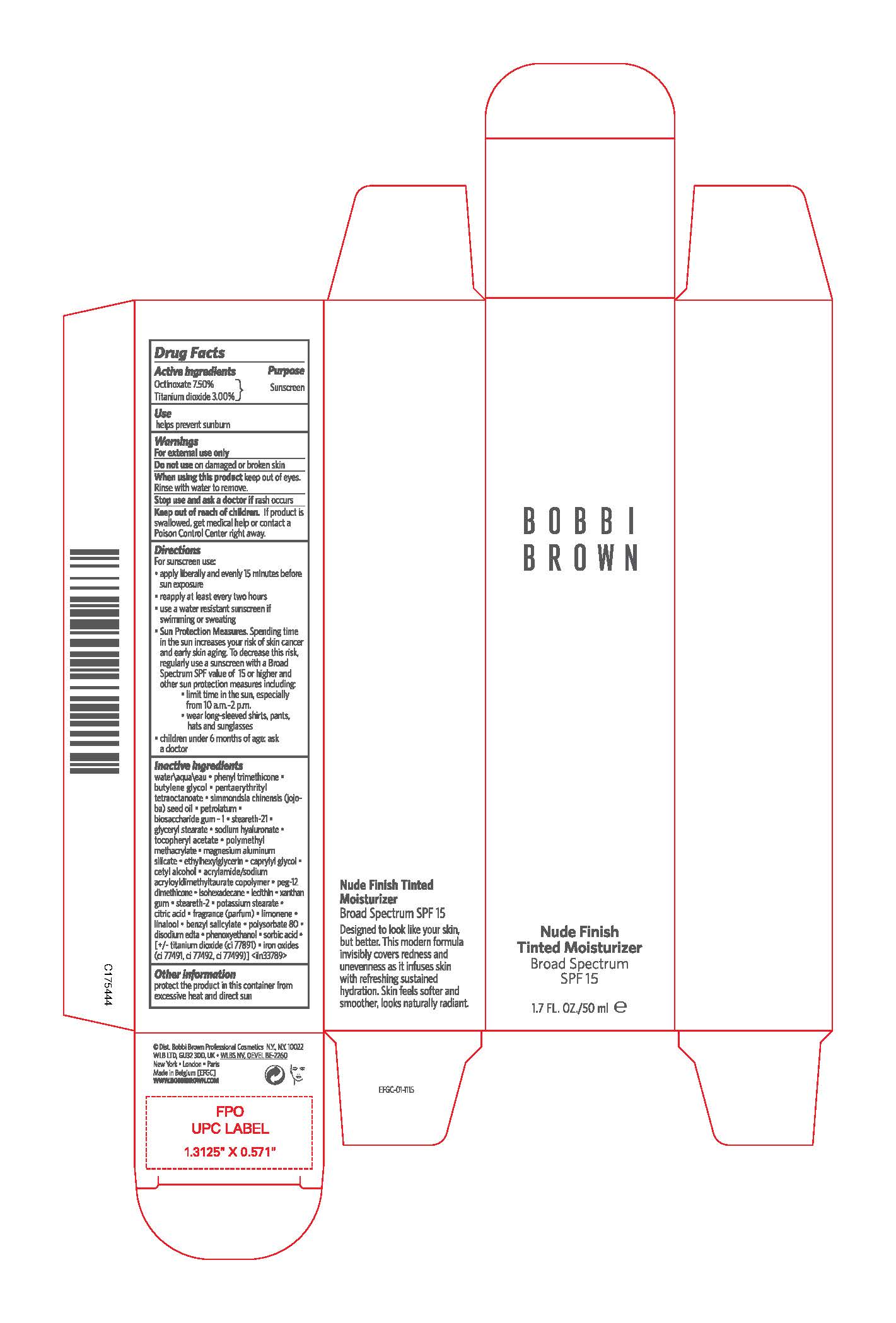
Label: BOBBI BROWN TINTED MOISTURIZER BROAD SPECTRUM SPF 15- octinoxate and titanium dioxide lotion
- NDC Code(s): 64141-027-01
- Packager: Bobbi Brown Professional Cosmetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau • phenyl trimethicone • butylene glycol • pentaerythrityl tetraoctanoate • simmondsia chinensis (jojoba) seed oil • petrolatum • biosaccharide gum - 1 • steareth-21 • glyceryl stearate • sodium hyaluronate • tocopheryl acetate • polymethyl methacrylate • magnesium aluminum silicate • ethylhexylglycerin • caprylyl glycol • cetyl alcohol • acrylamide/sodium acryloyldimethyltaurate copolymer • peg-12 dimethicone • isohexadecane • lecithin • xanthan gum • steareth-2 • potassium stearate • citric acid • fragrance (parfum) • limonene • linalool • benzyl salicylate • polysorbate 80 • disodium edta • phenoxyethanol • sorbic acid • [+/- titanium dioxide (ci 77891) • iron oxides (ci 77491, ci 77492, ci 77499)] <iln33789>
- Other information
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
BOBBI BROWN TINTED MOISTURIZER BROAD SPECTRUM SPF 15
octinoxate and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64141-027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTAERYTHRITYL TETRAETHYLHEXANOATE (UNII: XJ7052W897) JOJOBA OIL (UNII: 724GKU717M) PETROLATUM (UNII: 4T6H12BN9U) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) STEARETH-21 (UNII: 53J3F32P58) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETYL ALCOHOL (UNII: 936JST6JCN) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOHEXADECANE (UNII: 918X1OUF1E) XANTHAN GUM (UNII: TTV12P4NEE) STEARETH-2 (UNII: V56DFE46J5) POTASSIUM STEARATE (UNII: 17V812XK50) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) LINALOOL, (+/-)- (UNII: D81QY6I88E) BENZYL SALICYLATE (UNII: WAO5MNK9TU) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBIC ACID (UNII: X045WJ989B) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64141-027-01 1 in 1 CARTON 01/01/2001 05/01/2025 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2001 Labeler - Bobbi Brown Professional Cosmetics Inc. (627131279) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(64141-027) , pack(64141-027) , label(64141-027)