Label: NICOTINE TRANSDERMAL SYSTEM STEP 1- nicotine patch, extended release
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...view full title
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...
- NDC Code(s): 60429-443-14, 60429-444-14, 60429-445-14
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 43598-446, 43598-447, 43598-448
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
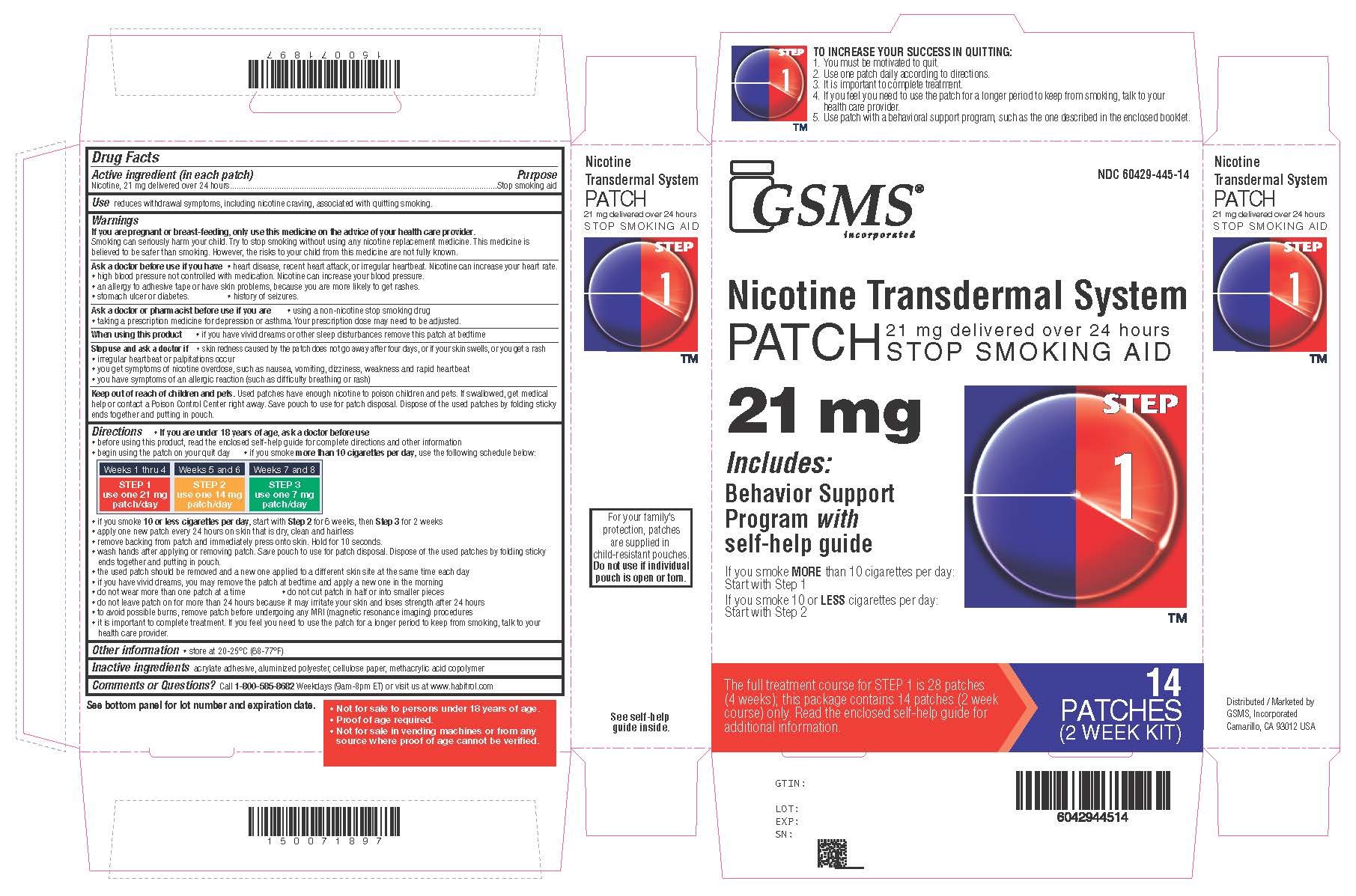
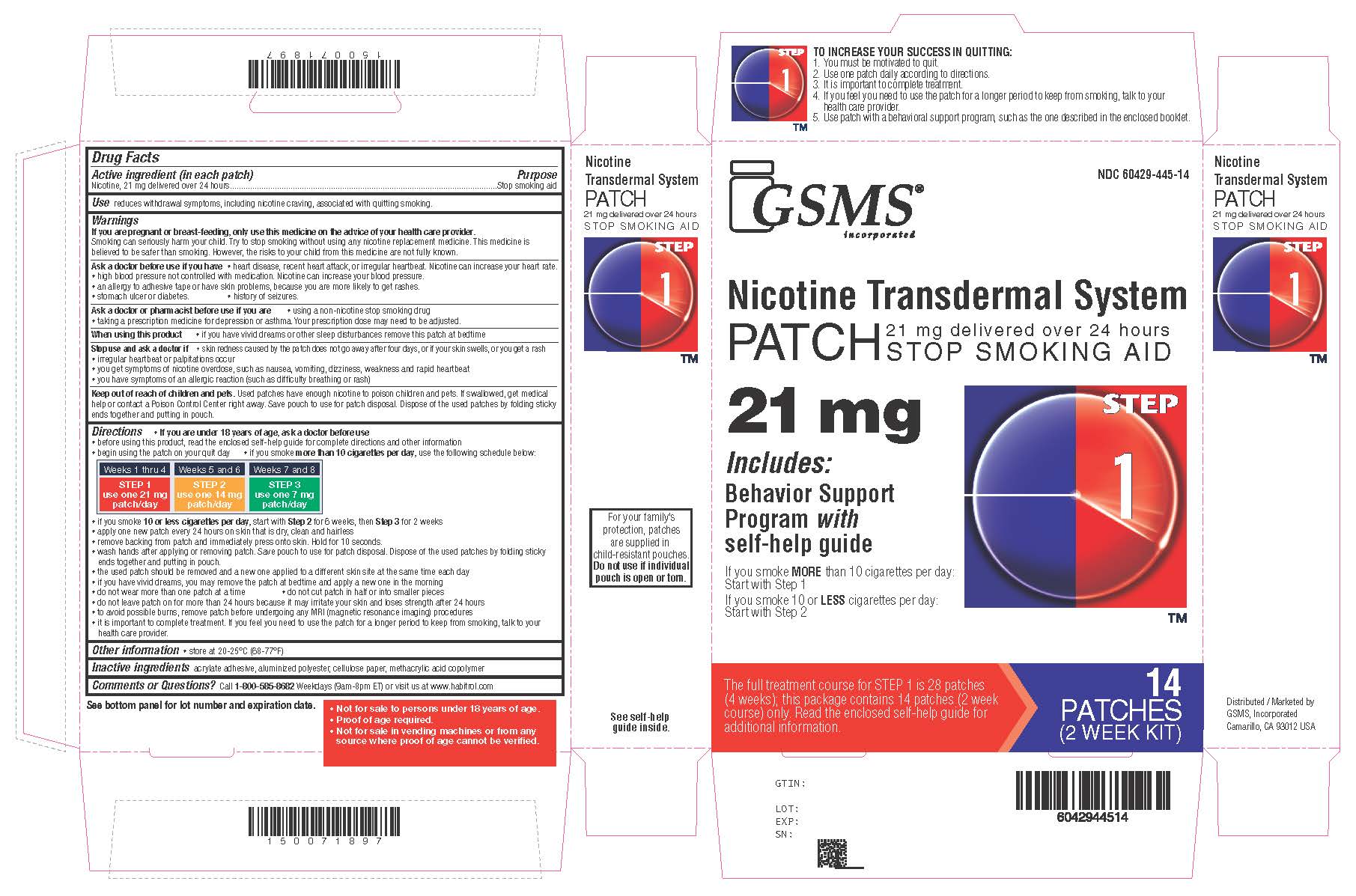
Active ingredient Step 1 (in each patch)
Nicotine, 21 mg delivered over 24 hours
-
Active ingredient Step 2 (in each patch)
Nicotine, 14 mg delivered over 24 hours
-
Active ingredient Step 3 (in each patch)
Nicotine, 7 mg delivered over 24 hours
-
Purpose
Stop smoking aid
-
Use
reduces withdrawal symptoms, including nicotine craving, associated with quitting smoking
-
WARNINGS SECTIONIf you are pregnant or breast-feeding, only use this medicine on the advice of your health care provider. Smoking can seriously harm your child. Try to stop smoking without using any ...
-
Directions
if you are under 18 years of age, ask a doctor before use - before using this product, read the enclosed self-help guide for complete directions and other information - begin using the patch on ...
-
Other information
store at 20-25°C (68-77°F)
-
Inactive ingredients
acrylate adhesive, aluminized polyester, cellulose paper, methacrylic acid copolymer
-
Questions or comments?
call - 1-800-585-8682 - Weekdays (9am-8pm ET) or visit us at www.habitrol.com - TO INCREASE YOUR SUCCESS IN QUITTING: 1. You must be motivated to quit. 2. Use one patch daily according to ...
-
Principal Display PanelNicotine Transdermal System PATCH - 21 mg delivered over 24 hours - STOP SMOKING AID - Includes:Behavior Support Programwithself-help guide - STEP 1 - IF YOU SMOKE - MORETHAN 10 CIGARETTES PER ...
-
Principal Display PanelNicotine Transdermal System PATCH - 14 mg delivered over 24 hours - STOP SMOKING AID - Includes:Behavior Support Programwithself-help guide - STEP 2 - IF YOU SMOKE - MORETHAN 10 CIGARETTES PER ...
-
Principal Display PanelNicotine Transdermal System PATCH - 7 mg delivered over 24 hours - STOP SMOKING AID - Includes:Behavior Support Programwithself-help guide - STEP 3 - FOR USE AFTER COMPLETING STEP 2
-
INGREDIENTS AND APPEARANCEProduct Information
View Labeling Archives for this drug
NICOTINE TRANSDERMAL SYSTEM STEP 1- nicotine patch, extended release
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...view full title
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...
Number of versions: 8
RxNorm
NICOTINE TRANSDERMAL SYSTEM STEP 1- nicotine patch, extended release
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...view full title
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...
Get Label RSS Feed for this Drug
NICOTINE TRANSDERMAL SYSTEM STEP 1- nicotine patch, extended release
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...view full title
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...
NDC Codes
NICOTINE TRANSDERMAL SYSTEM STEP 1- nicotine patch, extended release
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...view full title
NICOTINE TRANSDERMAL SYSTEM STEP 2- nicotine patch, ...