Label: NITROGLYCERIN ointment
- NDC Code(s): 21922-048-05
- Packager: Encube Ethicals Private Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NITROGLYCERIN OINTMENT safely and effectively. See full prescribing information for NITROGLYCERIN OINTMENT. NITROGLYCERIN ointment ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENitroglycerin ointment 0.4% is indicated for the treatment of moderate to severe pain associated with chronic anal fissure.
-
2 DOSAGE AND ADMINISTRATIONApply 1 inch of ointment (375 mg of ointment equivalent to 1.5 mg of nitroglycerin) intra-anally every 12 hours for up to 3 weeks. A finger covering, such as plastic-wrap, disposable surgical ...
-
3 DOSAGE FORMS AND STRENGTHSOintment, 0.4% w/w (4 mg /1 g) in 30 g tubes.
-
4 CONTRAINDICATIONS4.1 PDE5 inhibitor use - Administration of nitroglycerin ointment is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific ...
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular disorders - Venous and arterial dilatation as a consequence of nitroglycerin treatment including nitroglycerin ointment, can decrease venous blood returning to the heart and ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
7 DRUG INTERACTIONS7.1 PDE5 inhibitors - Phosphodiesterase type 5 (PDE5) inhibitors such as sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates. The time ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data on the use of nitroglycerin ointment intra-anally during pregnancy to determine a drug-associated risk of major birth defects, miscarriage or ...
-
10 OVERDOSAGENitroglycerin toxicity is generally mild. The estimated adult oral lethal dose of nitroglycerin is 200 mg to 1,200 mg. Infants may be more susceptible to toxicity from nitroglycerin. Consultation ...
-
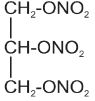
11 DESCRIPTIONNitroglycerin ointment, USP 0.4% is intended for intra-anal use. Nitroglycerin is 1,2,3,-propanetriol trinitrate, an organic nitrate vasodilator whose structural formula is as follows: and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nitroglycerin forms free radical nitric oxide (NO), which activates guanylate cyclase, resulting in an increase of guanosine 3',5'- monophosphate (cyclic GMP) in smooth ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Animal carcinogenicity studies with topically applied nitroglycerin have not been performed. Rats receiving up to 434 ...
-
14 CLINICAL STUDIESNitroglycerin ointment was evaluated in a 3-week double-blind, randomized, multi-center, placebo- controlled study. Patients with a painful chronic anal fissure for at least 6 weeks and moderate ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNitroglycerin ointment, USP 0.4% is available in 30 g (NDC 21922-048-05) aluminum tubes with polyethylene screw caps. Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for - Use) Interaction with PDE5 inhibitors - Advise patient not to use nitroglycerin ointment ...
-
PATIENT INFORMATIONNitroglycerin (nye" troe glis' er in) Ointment 0.4% IMPORTANT: For intra-anal use only - Read the Patient Information that comes with nitroglycerin ointment before you start using ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 21922-048-05 - Nitroglycerin Ointment, USP 0.4% 30 g - Rx only - Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information