Label: ACTHAR- repository corticotropin injection
ACTHAR- repository corticotropin injection
-
NDC Code(s):
63004-8710-1,
63004-8710-2,
63004-8711-1,
63004-8711-4, view more63004-8712-1, 63004-8712-4
- Packager: Mallinckrodt ARD LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ACTHAR® GEL safely and effectively. See full prescribing information for ACTHAR GEL.
ACTHAR GEL (repository corticotropin injection), for intramuscular or subcutaneous use
Initial U.S. Approval: 1952INDICATIONS AND USAGE
- Acthar Gel is indicated as monotherapy for the treatment of infantile spasms in infants and children under 2 years of age. (1.1)
- Acthar Gel is indicated for the treatment of exacerbations of multiple sclerosis in adults. (1.2)
- Acthar Gel may be used for the following disorders and diseases: rheumatic (1.3); collagen (1.4); dermatologic (1.5); allergic states (1.6); ophthalmic (1.7); respiratory (1.8); and edematous state. (1.9)
DOSAGE AND ADMINISTRATION
- Acthar Gel vial is for either intramuscular or subcutaneous injection. (2.1)
- Acthar Gel single-dose pre-filled SelfJect injector:
- Infantile spasms: doses must be administered intramuscularly using the Acthar gel vial. The recommended dose is 150 U/m2 divided into twice daily injections of 75 U/m2. After 2 weeks of treatment dosing should be gradually tapered and discontinued over a 2-week period. Acthar Gel single-dose pre-filled SelfJect injector is not to be used for the treatment of infantile spasms (2.2)
- Acute exacerbations of multiple sclerosis: daily intramuscular or subcutaneous doses of 80 to 120 units for 2-3 weeks may be administered. It may be necessary to taper the dose. (2.3)
- Other disorders and diseases: individualize dosing depending on the disease and patient. The usual dose is 40 to 80 units given intramuscularly or subcutaneously every 24 to 72 hours. It may be necessary to taper the dose. (2.4)
DOSAGE FORMS AND STRENGTHS
Injection available as:
CONTRAINDICATIONS
Acthar Gel is contraindicated:
- for intravenous administration (4)
- in infants under 2 years of age who have suspected congenital infections (4)
- with concomitant administration of live or live attenuated vaccines in patients receiving immunosuppressive doses of Acthar Gel (4)
- in patients with scleroderma, osteoporosis, systemic fungal infections, ocular herpes simplex, recent surgery, history of or the presence of a peptic ulcer, congestive heart failure, uncontrolled hypertension, primary adrenocortical insufficiency, adrenocortical hyperfunction, or sensitivity to proteins of porcine origin (4)
WARNINGS AND PRECAUTIONS
- Infections: Increased susceptibility to new infection and increased risk of exacerbation, dissemination or reactivation of latent infections. Signs and symptoms of infection may be masked. (5.1)
- Adrenal Insufficiency after Prolonged Therapy: Monitor for effects of hypothalamic-pituitary-adrenal axis suppression after stopping treatment. (5.2)
- Cushing's Syndrome: May occur after prolonged therapy. Monitor for signs and symptoms. (5.2)
- Elevated Blood Pressure, Salt and Water Retention, and Hypokalemia: Monitor blood pressure and sodium and potassium levels. (5.3)
- Masking of Symptoms of Other Underlying Disease/Disorders: Monitor patients for signs of other underlying disease/disorders that may be masked. (5.5)
- Gastrointestinal Perforation and Bleeding: There is a risk for gastric ulcers and bleeding. There is an increased risk of perforation in patients with certain GI disorders. Signs and symptoms may be masked. Monitor for signs of perforation and bleeding. (5.6)
- Behavioral and Mood Disturbances: May include euphoria, insomnia, mood swings, personality changes, severe depression and psychosis. Existing conditions may be aggravated. (5.7)
- Comorbid Diseases: Symptoms of diabetes and myasthenia gravis may be worsened with treatment. (5.8)
- Ophthalmic Effects: Monitor for cataracts, infections and glaucoma. (5.9)
- Immunogenicity Potential: Neutralizing antibodies with chronic administration may lead to a loss of endogenous ACTH activity. (5.10)
- Use in Patients with Hypothyroidism or Liver Cirrhosis: May result in an enhanced effect. (5.11)
- Negative Effects on Growth and Physical Development: Monitor pediatric patients on long term therapy. (5.12)
- Decrease in Bone Density: Monitor for osteoporosis in patients on long term therapy. (5.13)
ADVERSE REACTIONS
- Commonly reported postmarketing adverse reactions for Acthar Gel include injection site reaction, asthenic conditions (including fatigue, malaise, asthenia and lethargy), fluid retention (including peripheral swelling), insomnia, headache, and blood glucose increased. (6.2)
- The most common adverse reactions (5% or greater in the recommended twice daily dosing group) for the treatment of infantile spasms are increased risk of infections, convulsions, hypertension, irritability, and pyrexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mallinckrodt at 1-800-844-2830 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Acthar Gel may accentuate electrolyte loss associated with diuretics. (7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Infantile Spasms
1.2 Multiple Sclerosis
1.3 Rheumatic Disorders
1.4 Collagen Diseases
1.5 Dermatologic Diseases
1.6 Allergic States
1.7 Ophthalmic Diseases
1.8 Respiratory Diseases
1.9 Edematous State
2 DOSAGE AND ADMINISTRATION
2.1 Important Information
2.2 Recommended Dosage for Infantile Spasms in Infants and Children Under 2 Years of Age
2.3 Recommended Dosage for the Treatment of Acute Exacerbations in Adults with Multiple Sclerosis
2.4 Recommended Dosage for Other Indications for Adults and Children Over 2 Years of Age
2.5 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infections
5.2 Cushing's Syndrome and Adrenal Insufficiency Upon Withdrawal
5.3 Elevated Blood Pressure, Salt and Water Retention, and Hypokalemia
5.4 Vaccination
5.5 Masking Symptoms of Other Diseases
5.6 Gastrointestinal Perforation and Bleeding
5.7 Behavioral and Mood Disturbances
5.8 Comorbid Diseases
5.9 Ophthalmic Effects
5.10 Immunogenicity Potential
5.11 Use in Patients with Hypothyroidism or Liver Cirrhosis
5.12 Negative Effects on Growth and Physical Development
5.13 Decrease in Bone Density
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Possible Additional Steroidogenic Effects
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Infantile Spasms
Acthar Gel is indicated as monotherapy for the treatment of infantile spasms in infants and children under 2 years of age.
1.2 Multiple Sclerosis
Acthar Gel is indicated for the treatment of acute exacerbations of multiple sclerosis in adults. Controlled clinical trials have shown Acthar Gel to be effective in speeding the resolution of acute exacerbations of multiple sclerosis. However, there is no evidence that it affects the ultimate outcome or natural history of the disease.
1.3 Rheumatic Disorders
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in: Psoriatic arthritis; Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy); Ankylosing spondylitis.
1.4 Collagen Diseases
During an exacerbation or as maintenance therapy in selected cases of: systemic lupus erythematosus, systemic dermatomyositis (polymyositis).
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Information
Acthar Gel vial is intended for either intramuscular or subcutaneous injection.
Acthar Gel single-dose pre-filled SelfJect injector is for subcutaneous administration by adults (18 years of age and older) only. The single-dose pre-filled SelfJect injector should only be used to administer single doses of either 40 units or 80 units. For administration of doses other than 40 units or 80 units, use the Acthar Gel multi-dose vial.
2.2 Recommended Dosage for Infantile Spasms in Infants and Children Under 2 Years of Age
In the treatment of infantile spasms, Acthar Gel must be administered intramuscularly using the Acthar gel vial. Do not use the Acthar Gel single-dose pre-filled SelfJect injector for the treatment of infantile spasms. The recommended regimen is a daily dose of 150 U/m2 (divided into twice daily intramuscular (IM) injections of 75 U/m2) administered over a 2-week period. Dosing with Acthar Gel should then be gradually tapered over a 2-week period to avoid adrenal insufficiency. The following is one suggested tapering schedule: 30 U/m2 in the morning for 3 days; 15 U/m2 in the morning for 3 days; 10 U/m2 in the morning for 3 days; and 10 U/m2 every other morning for 6 days.
Acthar Gel is typically dosed based on body surface area (BSA). For calculation of body surface area, use the following formula:

2.3 Recommended Dosage for the Treatment of Acute Exacerbations in Adults with Multiple Sclerosis
The recommended dose is daily intramuscular or subcutaneous doses of 80 to 120 units for 2-3 weeks for acute exacerbations.
Dosage should be individualized according to the medical condition of each patient. Frequency and dose of the drug should be determined by considering the severity of the disease and the initial response of the patient.
Although drug dependence does not occur, sudden withdrawal of Acthar Gel after prolonged use may lead to adrenal insufficiency or recurrent symptoms which make it difficult to stop the treatment. It may be necessary to taper the dose and increase the injection interval to gradually discontinue the medication.
2.4 Recommended Dosage for Other Indications for Adults and Children Over 2 Years of Age
Dosage should be individualized according to the disease under treatment and the general medical condition of each patient. Frequency and dose of the drug should be determined by considering severity of the disease and the initial response of the patient.
The usual dose of Acthar Gel is 40 to 80 units given intramuscularly or subcutaneously every 24 to 72 hours.
Although drug dependence does not occur, sudden withdrawal of Acthar Gel after prolonged use may lead to adrenal insufficiency or recurrent symptoms which make it difficult to stop the treatment. It may be necessary to taper the dose and increase the injection interval to gradually discontinue the medication.
2.5 Preparation and Administration
Visually inspect the liquid for particulate matter and discoloration prior to administration. Acthar Gel must not be injected if the solution is cloudy or contains particulate matter.
Acthar Gel Multi-Dose Vial
- Warm to room temperature before using.
- Take caution to not over-pressurize the vial prior to withdrawing the product.
Acthar Gel Single-Dose Pre-filled SelfJect Injector
Preparation
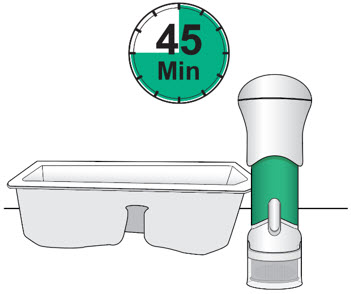
- Prior to injection, remove from the refrigerator and sealed tray and allow to sit for 45 minutes to warm to room temperature.
Administration
- Read the FDA-approved Instructions for Use carefully before administering.
- Administer by subcutaneous injection only. Acthar Gel single-dose pre-filled SelfJect injector is not for intramuscular injection.
- Inject in the upper thigh, abdomen, or back of arm. Avoid injecting within 1 inch of navel, knee, or groin area.
- Avoid areas with scars, tattoos, warts, birthmarks, or stretch marks, or where the skin is irritated.
- Rotate injection sites. Do not use the same site more than one time per week.
-
3 DOSAGE FORMS AND STRENGTHS
Injection available as:
400 USP Units/5 mL (80 USP Units/mL) in a multi-dose vial for subcutaneous or intramuscular injection.
40 USP Units/0.5 mL in a single-dose pre-filled SelfJect injector for subcutaneous injection.
80 USP Units/mL in a single-dose pre-filled SelfJect injector for subcutaneous injection.
Acthar Gel (repository corticotropin injection) is a clear light amber solution mobile at room temperature.
-
4 CONTRAINDICATIONS
Acthar Gel is contraindicated:
- for intravenous administration.
- in infants under 2 years of age who have suspected congenital infections.
- with concomitant administration of live or live attenuated vaccines in patients receiving immunosuppressive doses of Acthar Gel.
- in patients with scleroderma, osteoporosis, systemic fungal infections, ocular herpes simplex, recent surgery, history of or the presence of a peptic ulcer, congestive heart failure, uncontrolled hypertension, primary adrenocortical insufficiency, adrenocortical hyperfunction, or sensitivity to proteins of porcine origin.
-
5 WARNINGS AND PRECAUTIONS
The adverse effects of Acthar Gel are related primarily to its steroidogenic effects. Not all of the adverse events described below have been seen after treatment with Acthar Gel, but they might be expected to occur because they are steroidogenic effects [see Adverse Reactions (6.3)].
5.1 Infections
Acthar Gel may increase the risks related to infections with any pathogen, including viral, bacterial, fungal, protozoan or helminthic infections. Patients with latent tuberculosis or tuberculin reactivity should be observed closely, and if therapy is prolonged, chemoprophylaxis should be instituted.
5.2 Cushing's Syndrome and Adrenal Insufficiency Upon Withdrawal
Treatment with Acthar Gel can cause hypothalamic-pituitary-adrenal (HPA) axis suppression and Cushing's syndrome. These conditions should be monitored especially with chronic use.
Suppression of the HPA may occur following prolonged therapy with the potential for adrenal insufficiency after withdrawal of the medication. Patients should be monitored for signs of insufficiency such as weakness, hyperpigmentation, weight loss, hypotension and abdominal pain.
The symptoms of adrenal insufficiency in infants treated for infantile spasms can be difficult to identify. The symptoms are non-specific and may include anorexia, fatigue, lethargy, weakness, excessive weight loss, hypotension and abdominal pain. It is critical that parents and caregivers be made aware of the possibility of adrenal insufficiency when discontinuing Acthar Gel and should be instructed to observe for, and be able to recognize, these symptoms [see Patient Counseling Information (17)].
The recovery of the adrenal gland may take from days to months so patients should be protected from the stress (e.g., trauma or surgery) by the use of corticosteroids during the period of stress.
The adrenal insufficiency may be minimized by tapering of the dose when discontinuing treatment.
Signs or symptoms of Cushing's syndrome may occur during therapy but generally resolve after therapy is stopped. Patients should be monitored for these signs and symptoms such as deposition of adipose tissue in characteristics sites (e.g., moon face, truncal obesity), cutaneous striae, easy bruisability, decreased bone mineralization, weight gain, muscle weakness, hyperglycemia, and hypertension.
5.3 Elevated Blood Pressure, Salt and Water Retention, and Hypokalemia
Acthar Gel can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium and calcium. Dietary salt restriction and potassium supplementation may be necessary. Caution should be used in the treatment of patients with hypertension or renal insufficiency. Acthar Gel is contraindicated in patients with congestive heart failure [see Contraindications (4)].
5.4 Vaccination
Administration of live or live attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of Acthar Gel. Killed or inactivated vaccines may be administered; however, the response to such vaccines can not be predicted. Other immunization procedures should be undertaken with caution in patients who are receiving Acthar Gel, especially when high doses are administered, because of the possible hazards of neurological complications and lack of antibody response.
5.5 Masking Symptoms of Other Diseases
Acthar Gel often acts by masking symptoms of other diseases/disorders without altering the course of the other disease/disorder. Patients should be monitored carefully during and for a period following discontinuation of therapy for signs of infection, abnormal cardiac function, hypertension, hyperglycemia, change in body weight and fecal blood loss.
5.6 Gastrointestinal Perforation and Bleeding
Acthar Gel can cause GI bleeding and gastric ulcer. There is also an increased risk for perforation in patients with certain gastrointestinal disorders. Signs of gastrointestinal perforation, such as peritoneal irritation, may be masked by the therapy. Use caution where there is the possibility of impending perforation, abscess or other pyogenic infections, diverticulitis, fresh intestinal anastomoses, and active or latent peptic ulcer.
5.7 Behavioral and Mood Disturbances
Use of Acthar Gel may be associated with central nervous system effects ranging from euphoria, insomnia, irritability (especially in infants), mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated. These effects are reversible once Acthar Gel therapy is stopped.
5.8 Comorbid Diseases
Patients with a comorbid disease may have that disease worsened. Caution should be used when prescribing Acthar Gel in patients with diabetes and myasthenia gravis.
5.9 Ophthalmic Effects
Prolonged use of Acthar Gel may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves and may enhance the establishment of secondary ocular infections due to fungi and viruses.
5.10 Immunogenicity Potential
Acthar Gel is immunogenic. Limited available data suggest that a patient may develop antibodies to Acthar Gel after chronic administration and loss of endogenous ACTH and Acthar Gel activity. Prolonged administration of Acthar Gel may increase the risk of hypersensitivity reactions. Cases of anaphylaxis have been reported in the postmarketing setting. Use in patients with sensitivity to porcine protein is contraindicated, and the possibility of sensitivity should be considered during the course of treatment should symptoms arise.
5.11 Use in Patients with Hypothyroidism or Liver Cirrhosis
There is an enhanced effect in patients with hypothyroidism and in those with cirrhosis of the liver.
5.12 Negative Effects on Growth and Physical Development
Long-term use of Acthar Gel may have negative effects on growth and physical development in pediatric patients. Changes in appetite are seen with Acthar Gel therapy, with the effects becoming more frequent as the dose or treatment period increases. These effects are reversible once Acthar Gel therapy is stopped. Growth and physical development of pediatric patients on prolonged therapy should be carefully monitored.
5.13 Decrease in Bone Density
Decrease in bone formation and an increase in bone resorption both through an effect on calcium regulation (i.e., decreasing absorption and increasing excretion) and inhibition of osteoblast function may occur. These, together with a decrease in the protein matrix of the bone (secondary to an increase in protein catabolism) and reduced sex hormone production, may lead to inhibition of bone growth in children and adolescents and to the development of osteoporosis at any age. Special consideration should be given to patients at increased risk of osteoporosis (i.e., postmenopausal women) before initiating therapy, and bone density should be monitored in patients on long term therapy.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infections [see Warnings and Precautions (5.1)]
- Cushing's Syndrome and Adrenal Insufficiency Upon Withdrawal [see Warnings and Precautions (5.2)]
- Elevated Blood Pressure, Salt and Water Retention, and Hypokalemia [see Warnings and Precautions (5.3)]
- Masking Symptoms of Other Diseases [see Warnings and Precautions (5.5)]
- Gastrointestinal Perforation and Bleeding [see Warnings and Precautions (5.6)]
- Behavioral and Mood Disturbances [see Warnings and Precautions (5.7)]
- Ophthalmic Effects [see Warnings and Precautions (5.9)]
- Immunogenicity Potential [see Warnings and Precautions (5.10)]
- Negative Effects on Growth and Physical Development [see Warnings and Precautions (5.12)]
- Decrease in Bone Density [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Infants and Children Under 2 Years of Age
While the types of adverse reactions seen in infants and children under age 2 treated for infantile spasms are similar to those seen in older patients, their frequency and severity may be different due to the very young age of the infant, the underlying disorder, the duration of therapy and the dosage regimen. Below is a summary of adverse reactions specifically tabulated from source data derived from retrospective chart reviews and clinical trials in children under 2 years of age treated for infantile spasms. The number of patients in controlled trials at the recommended dose was too few to provide meaningful incidence rates or to permit a meaningful comparison to the control groups. The most common adverse reactions (5% or greater in the recommended twice daily dosing group) for the treatment of infantile spasms are increased risk of infections, convulsions, hypertension, irritability, and pyrexia.
TABLE: Incidence (%) of Adverse Reactions Occurring in ≥2% of Infants and Children Under 2 Years of Age Treated with Acthar Gel Adverse Reactions Recommended
75 U/m2 twice daily
n=122, (%)150 U/m2 once daily
n=37 (%)- *
- Specific infections that occurred at ≥2% were candidiasis, otitis media, pneumonia and upper respiratory tract infections.
- †
- In the treatment of infantile spasms, other types of seizures/convulsions may occur because some patients with infantile spasms progress to other forms of seizures (for example, Lennox-Gastaut Syndrome). Additionally, the spasms sometimes mask other seizures and once the spasms resolve after treatment, the other seizures may become visible.
Cardiac disorders Cardiac Hypertrophy 3 0 Endocrine disorders Cushingoid 3 22 Gastrointestinal disorders Diarrhea 3 14 Vomiting 3 5 Constipation 0 5 General disorders and administration site conditions Irritability 7 19 Pyrexia 5 8 Infections and infestations Infection* 20 46 Investigations Weight gain 1 3 Metabolism and nutrition disorders Increased appetite 0 5 Decreased appetite 3 3 Nervous system disorders Convulsion† 12 3 Respiratory, thoracic and mediastinal disorders Nasal Congestion 1 5 Skin and subcutaneous tissue disorders Acne 0 14 Rash 0 8 Vascular disorders Hypertension 11 19 These adverse reactions may also be seen in adults and children over 2 years of age when treated for other purposes and with different doses and regimens.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Acthar Gel.
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic Reactions
Allergic responses have presented as dizziness, nausea, and anaphylaxis (anaphylactic shock, hypotension, respiratory compromise, urticaria, edema).
Cardiovascular
Necrotizing angitis (adults only), congestive heart failure, atrial fibrillation, and palpitations.
General Disorders and Administration Site Conditions
Injection site reaction and asthenic conditions (including fatigue, malaise, asthenia, and lethargy).
6.3 Possible Additional Steroidogenic Effects
Based on steroidogenic effects of Acthar Gel certain adverse events may be expected due to the pharmacological effects of corticosteroids. The adverse events that may occur but have not been reported for Acthar Gel are:
Dermatologic
Impaired wound healing, petechiae and ecchymoses, and suppression of skin test reactions.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on Acthar Gel's pharmacological effect of stimulating an endogenous steroid response [see Clinical Pharmacology ( 12.1)], Acthar Gel may cause fetal harm when administered to a pregnant woman. The published literature on systemic corticosteroid use during pregnancy, which may be relevant, suggests potential concerns. Intrauterine growth restriction, decreased birth weight, and preterm birth have been reported with maternal use of corticosteroids; however, the underlying maternal condition may also contribute to these risks. Hypoadrenalism has also been reported in infants after high-dose and/or long-term use of corticosteroids during pregnancy (see Clinical Considerations). The potential adverse developmental effects of Acthar Gel have not been adequately assessed in animals.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal-Neonatal Adverse Reactions
Hypoadrenalism has been reported in infants born to mothers treated with systemic corticosteroids during pregnancy. Infants born to mothers treated with Acthar Gel should be carefully observed for signs of hypoadrenalism, such as poor feeding, irritability, weakness, and vomiting, and managed accordingly [see Warnings and Precautions (5.2)].
8.2 Lactation
Risk Summary
There are no available data on the presence of corticotropin in either human or animal milk, the effects on the breastfed infant, or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Acthar Gel and any potential adverse effects on the breastfed infant from Acthar Gel or from the underlying maternal condition.
8.4 Pediatric Use
Acthar Gel is indicated as monotherapy for the treatment of infantile spasms in infants and children less than 2 years of age. Both serious and other adverse reactions can occur in this population [see Warnings and Precautions (5) and Adverse Reactions (6.1)].
The efficacy of Acthar Gel for the treatment of infantile spasms in infants and children less than 2 years of age was evaluated in a randomized, single blinded (video EEG interpreter blinded) clinical trial and an additional active control supportive trial [see Clinical Studies (14)]. A responding patient was defined as having both complete cessation of spasms and elimination of hypsarrhythmia.
Safety in the pediatric population for infantile spasms was evaluated by retrospective chart reviews and data from non-sponsor conducted clinical trials [see Adverse Reactions (6.1)]. While the types of adverse reactions seen in infants and children under 2 years of age treated for infantile spasms are similar to those seen in older patients, their frequency and severity may be different due to the very young age of the infant, the underlying disorder, the duration of therapy and the dosage regimen. Effects on growth are of particular concern [see Warnings and Precautions (5.12)]. Serious adverse reactions observed in adults may also occur in children [see Warnings and Precautions (5)].
-
11 DESCRIPTION
Acthar Gel is a naturally sourced complex mixture of adrenocorticotropic hormone analogs and other pituitary peptides. The Acthar Gel manufacturing process converts the initial porcine pituitary extract with low ACTH content into a mixture having modified porcine ACTH and other related peptide analogs solubilized in gelatin. A major component in the formulated complex mixture is N-25 deamidated porcine ACTH (1-39).
Acthar Gel is supplied as a sterile preparation in 16% gelatin to provide a prolonged release after intramuscular or subcutaneous injection. Acthar Gel also contains 0.5% phenol, not more than 0.1% cysteine (added), sodium hydroxide and/or acetic acid to adjust pH and Water for Injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of Acthar Gel in the treatment of infantile spasms is unknown.
Acthar Gel and endogenous ACTH stimulate the adrenal cortex to secrete cortisol, corticosterone, aldosterone, and a number of weakly androgenic substances. Prolonged administration of large doses of Acthar Gel induces hyperplasia and hypertrophy of the adrenal cortex and continuous high output of cortisol, corticosterone and weak androgens. The release of endogenous ACTH is under the influence of the nervous system via the regulatory hormone released from the hypothalamus and by a negative corticosteroid feedback mechanism. Elevated plasma cortisol suppresses ACTH release.
Acthar Gel is also reported to bind to melanocortin receptors.
The trophic effects of endogenous ACTH and Acthar Gel on the adrenal cortex are not well understood beyond the fact that they appear to be mediated by cyclic AMP.
12.3 Pharmacokinetics
ACTH rapidly disappears from the circulation following its intravenous administration; in people, the plasma half-life is about 15 minutes. The pharmacokinetics of Acthar Gel have not been adequately characterized.
The maximal effects of a trophic hormone on a target organ are achieved when optimal amounts of hormone are acting continuously. Thus, a fixed dose of Acthar Gel will demonstrate a linear increase in adrenocortical secretion with increasing duration for the infusion.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The effectiveness of Acthar Gel as a treatment for infantile spasms was demonstrated in a single blinded (video EEG interpreter blinded) clinical trial in which patients were randomized to receive either a 2-week course of treatment with Acthar Gel (75 U/m2 intramuscular twice daily) or prednisone (1 mg/kg by mouth twice daily). The primary outcome was a comparison of the number of patients in each group who were treatment responders, defined as a patient having complete suppression of both clinical spasms and hypsarrhythmia on a full sleep cycle video EEG performed 2 weeks following treatment initiation, rated by an investigator blinded to treatment. Thirteen of 15 patients (86.7%) responded to Acthar Gel as compared to 4 of 14 patients (28.6%) given prednisone (p<0.002). The 2-week treatment was followed by a 2-week period of taper. Nonresponders to the prednisone treatment were eligible to receive Acthar Gel treatment. Seven of 8 patients (87.5%) responded to Acthar Gel after not responding to prednisone. Similarly, the 2 nonresponder patients from the Acthar Gel treatment were eligible to receive treatment with prednisone. One of the 2 patients (50%) responded to the prednisone treatment after not responding to Acthar Gel.
A supportive single-blind, randomized clinical trial comparing high-dose, long-duration treatment (150 U/m2 once daily for 3 weeks, n=30) of Acthar Gel with low-dose, short-duration treatment (20 U once daily for 2 weeks, n=29) for the treatment of infantile spasms was also evaluated in infants and children less than 2 years of age. Nonresponders (defined as in the previously described study) in the low-dose group received a dose escalation at 2 weeks to 30 U once daily. Nominal statistical superiority of the high dose treatment, as compared to the low dose treatment, was observed for cessation of spasms but not for the resolution of hypsarrhythmia.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Acthar Gel (repository corticotropin injection) is a clear light amber solution mobile at room temperature. Acthar Gel is supplied in the following configurations:
Strength Package size NDC number 80 USP Units/mL 5 mL multi-dose vial (1 count) 63004-8710-1 40 USP Units/0.5 mL Single-dose pre-filled SelfJect injector (4 count) 63004-8712-4 80 USP Units/mL Single-dose pre-filled SelfJect injector (4 count) 63004-8711-4 16.2 Storage and Handling
Store Acthar Gel vial and Acthar Gel single-dose pre-filled SelfJect injector under refrigeration between 2°C to 8°C (36°F to 46°F) in the carton to protect from light.
After removing the single-dose pre-filled SelfJect injector from the refrigerator, it can be stored at room temperature between 20°C to 25°C (68°F to 77°F) for up to 24 hours. Do not heat, freeze, or put the Acthar Gel single-dose pre-filled SelfJect injector into direct sunlight.
Dispense single-dose pre-filled SelfJect injectors in the original sealed carton with the enclosed Instructions for Use.
-
17 PATIENT COUNSELING INFORMATION
Advise caretakers of patients with infantile spasms to read the FDA-approved patient labeling (Medication Guide [for infantile spasms]).
Advise caretakers or patients 18 years of age or older to read the FDA-approved Instructions for Use when administering Acthar Gel single-dose pre-filled SelfJect injector.
Use and Monitoring
Patients should be instructed to take Acthar Gel only as prescribed. They should not stop treatment suddenly unless instructed by their healthcare provider to do so.
Patients, their caregivers and families should be advised as to the importance of the need for careful monitoring while on and during titration from Acthar Gel treatment and the importance of not missing scheduled doctor's appointments.
Infections
Advise patients, their caregivers, and families to contact their healthcare provider if the patient develops an infection or fever. Inform patients and caregivers that a fever may not necessarily be present during infection and to try to limit contact with other people with infections to minimize the risk of infection while taking Acthar Gel [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Cushing's Syndrome and Adrenal Insufficiency Upon Withdrawal
Advise patients to contact their healthcare provider if they develop signs or symptoms of Cushing's Syndrome, such as fat increases in the face (moon face) or trunk area, cutaneous striae, easy bruisability, weight gain, muscle weakness, hyperglycemia, and hypertension [see Warnings and Precautions (5.2)].
Inform patients that adrenal insufficiency can occur upon withdrawal of Acthar Gel. Advise patients to contact their healthcare provider if they develop weakness, hyperpigmentation, weight loss, hypotension, or abdominal pain. Advise caregivers of patients with infantile spasms that symptoms of adrenal insufficiency may be difficult to identify and that additional symptoms may include anorexia, fatigue, or lethargy [see Warnings and Precautions (5.2)].
Elevated Blood Pressure, Salt and Water Retention, and Hypokalemia
Advise patients, their caregivers and families to contact their healthcare provider if the patient experiences an increase in blood pressure or water retention [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
Vaccinations
Advise patients and caregivers of patients to not to be vaccinated with live or live attenuated vaccines during treatment with Acthar Gel. Additionally, other immunization procedures in patients or in family members who will be in contact with the patient should be undertaken with caution while the patient is taking Acthar Gel [see Warnings and Precautions (5.4)].
Masking Symptoms of Other Diseases
Inform patients, their caregivers, and families that Acthar Gel may mask symptoms of other diseases/disorders without altering the course of the other disease/disorder. Monitor the patient carefully during and for a period following discontinuation of therapy and inform their healthcare provider if signs of infection, abnormal cardiac function, hypertension, hyperglycemia, change in body weight, or fecal blood loss occur [see Warnings and Precautions (5.5)].
Gastrointestinal Perforation and Bleeding
Advise patients, their caregivers and families to contact their healthcare provider if the patient or the caregiver notices blood or a change in color of the patient's stool [see Warnings and Precautions (5.6)].
Behavioral and Mood Disturbances
Inform patients, their caregivers and families that signs of irritability, sleep disturbances, mood swings, personality changes, or severe depression may occur. These effects are reversible once Acthar Gel therapy is stopped [see Warnings and Precautions (5.7) and Adverse Reactions (6.1)].
Negative Effects on Growth and Physical Development
Advise patients, their caregivers and families that changes in appetite, most often leading to weight gain, are seen with Acthar Gel therapy, becoming more frequent as the dose or treatment period increases. These effects are reversible once Acthar Gel therapy is stopped [see Warnings and Precautions (5.12) and Adverse Reactions (6.1)].
Decrease in Bone Density
Advise patients, their caregivers and families that prolonged use of Acthar Gel may inhibit skeletal growth in children and adolescents and may cause osteoporosis and decreased bone density at any age. If prolonged use is necessary, Acthar Gel should be given intermittently along with careful observation [see Warnings and Precautions (5.13) and Adverse Reactions (6.1)].
Infantile Spasms Additional Information
Inform caregivers that, in the treatment of infantile spasms, other types of seizures may occur because some patients with infantile spasms progress to other forms of seizures (for example, Lennox-Gastaut Syndrome). Additionally, the spasms sometimes mask other seizures and once the spasms resolve after treatment with Acthar Gel, the other seizures may become visible. Advise parents and caregivers to inform the patient's healthcare provider of any new onset of seizures so that appropriate management can then be instituted [see Adverse Reactions (6.1)].
Pregnancy
Advise pregnant women of the potential risk to a fetus [see Use in Specific Populations ( 8.1)].
Mallinckrodt, the "M" brand mark, SelfJect, the Mallinckrodt Pharmaceuticals logo and other brands are trademarks of a Mallinckrodt company. © 2024 Mallinckrodt.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Acthar® Gel (AK-thar jel)
(repository corticotropin injection)This Medication Guide provides information only about the use of Acthar Gel for the treatment of Infantile Spasms. If your doctor prescribes Acthar Gel for you or your child for any other reason, talk to your doctor for information about how this medicine is used to treat your medical condition.
Read this Medication Guide before your child receives Acthar Gel and each time you refill your child's prescription. There may be new information. This Medication Guide does not take the place of talking with your doctor about your child's medical condition or treatment.
What is the most important information I should know about Acthar Gel?
Acthar Gel can cause serious side effects including:
- 1.
-
Increased risk of infections.
Acthar Gel is a medicine that can affect your child's immune system. When your child is taking Acthar Gel, it can lower the ability of your child's immune system to fight infections.
Acthar Gel may:
- make your child more likely to get new infections
- worsen an infection that your child already has
- cause an inactive infection to become active, such as tuberculosis (TB)
- an infection or signs of an infection, such as:
- fever
- cough
- vomiting
- diarrhea
- other signs of flu or illness
- a family member with an infection or signs of an infection
- stay away from people who are sick or who have infections
- tell your doctor right away if your child has any sign of infection such as:
- fever (but your child may not have a fever with an infection)
- cough
- vomiting
- diarrhea or
- other signs of illness or flu and
- any open cuts or sores on his or her body
- 2.
-
Effects on the adrenal gland after stopping Acthar Gel.
When your child stops taking Acthar Gel, his or her body may not produce enough of a hormone called cortisol on its own (adrenal insufficiency). Your child may need to take steroid medicine to protect the body until the adrenal gland recovers and is working well again, especially to protect the body if they have surgery or trauma. Do not stop giving your child injections of Acthar Gel without talking to your doctor first.
Your doctor will tell you when and how to slowly stop giving the injections to avoid serious side effects.
While slowly stopping your child's injections of Acthar Gel or after you stop giving the injections, call your doctor right away if your child has any of the following:
- appears weak
- loses weight or has a decrease in appetite
- appears tired or lacking energy
- appears pale
- has stomach pain
- appears sick or is with a fever
- 3.
-
Effects on the adrenal gland while taking Acthar Gel.
When your child is taking Acthar Gel, his or her adrenal gland may produce too much cortisol. This can cause symptoms of Cushing's syndrome. Cushing's syndrome is more common in children who take Acthar Gel for a long time.
Symptoms of Cushing's syndrome include:
- increased upper body fat around the neck, but not the arms and legs
- weight gain
- rounded or "moon" face
- thin skin, easy bruising, and stretch marks on thighs, belly and trunk
- slowed growth rates in children
- weak bones (osteoporosis)
- increased blood pressure. Your doctor may check your child's blood pressure during treatment. If your child's blood pressure increases, your doctor may talk with you about possible treatment choices.
- too much water in the body (water retention), increased amount of body salts, and low potassium in the blood. Acthar Gel may cause your child to have an increased amount of body salts and water that stays in the body, and may lower the amount of potassium in your child's blood. Follow your doctor's instructions about if you need to decrease your child's salt intake or if you need to feed your child foods high in potassium.
- 4.
-
Your child should not receive certain vaccines during treatment with Acthar Gel.
Your child may receive killed or inactivated vaccines while receiving Acthar Gel. Before your child receives any vaccines, talk to your doctor about which vaccines are safe for your child. Certain vaccines could cause your child to have serious side effects, or the vaccine may not be effective.
- 5.
-
Hiding (masking) symptoms of other conditions or diseases.
It may be more difficult for your doctor to diagnose other conditions or diseases in your child during treatment with Acthar Gel. During treatment and after treatment ends, tell your doctor if your child has:
- any signs or symptoms of infection. See number 1 of this section in the Medication Guide.
- changes in body weight
- bloody or black tarry stool
- vomiting
- stomach pain
- excessive tiredness
- increased thirst
- fast heart rate
- difficulty breathing
- 6.
-
Stomach and intestinal problems. Acthar Gel may cause bleeding of the stomach or intestine.
Your child has an increased risk for bleeding from the stomach or having a stomach ulcer. Tell your doctor if your child has any pain in the stomach area (abdominal pain), vomits blood, or has bloody or black stools.
- 7.
-
Changes in mood and behavior.
During treatment with Acthar Gel your child may be irritable, have rapid changes in his or her mood, be depressed, have other changes in his or her behavior, or have trouble sleeping.
Tell your doctor if your child has any of the side effects or symptoms listed above.
What is Acthar Gel?
Acthar Gel is a prescription medicine that is used to treat infantile spasms in infants and children under 2 years of age.
What should I tell my doctor before my child takes Acthar Gel?
Before your child takes Acthar Gel, read the section above "What is the most important information I should know about Acthar Gel?" and tell your doctor if your child has:
- an infection
- diabetes
- heart problems
- kidney problems
- stomach or intestinal problems
- thyroid problems
- liver problems
- neuromuscular problems
- convulsions or seizures
- had exposure to someone with Tuberculosis (TB)
- a previous allergic reaction such as hives, itching or trouble breathing, to Acthar Gel or pork products
- had recent surgery
- had a recent vaccination or is scheduled to receive a vaccination
- a family member who is receiving vaccinations
Tell your doctor about all the medicines your child takes, including prescription and non- prescription medicines, vitamins and herbal supplements. Do not start giving a new medicine to your child without first speaking to your doctor.
How should I give Acthar Gel to my child?
Acthar Gel is given as an injection into the muscle. Do not inject it under the skin, into a vein, or give it to your child by mouth.
- Inject Acthar Gel exactly as your doctor tells you. Your doctor will tell you where to give the injection, how much to give, how often and when to give it to your child.
- Do not use Acthar Gel until your doctor has taught you how to give the injection to your child.
- To give Acthar Gel:
- Take the bottle from the refrigerator. Do not open the bottle or pry the cap (rubber stopper) off.
- Warm the contents by rolling the bottle between your hands for a few minutes.
- Wash your hands.
- Prepare the skin where you are going to give the injection by wiping it with a new sterile alcohol wipe. Before giving the injection, look at the site prepared for the injection and make sure that it no longer looks wet. A wet site can cause burning.
- Wipe the top of the vial rubber stopper with a new sterile alcohol wipe.
- Use a new sterile needle and syringe to draw up the amount of Acthar Gel the doctor has told you to use.
- Give the injection the way the doctor has instructed you.
- Return the bottle to the refrigerator as soon as possible.
Keep all of your child's follow-up appointments with your doctor
- It is important for you to tell your doctor if your child's spasms continue or change in any way during treatment or after treatment has stopped so that they can monitor your child's progress.
Infantile Spasms sometimes hides (masks) other seizures your child or infant may have. Once treated with Acthar Gel, the Infantile Spasms symptoms may disappear. This may allow the other seizures to become visible for the first time. Tell your child's doctor right away if you see a change in your child's seizures/spasms.
What are the possible side effects of Acthar Gel?
Acthar Gel can cause serious side effects.
- See "What is the most important information I should know about Acthar Gel."
- Acthar Gel may make certain other medical conditions worse, such as diabetes (may increase blood sugar).
- Eye problems. Your child can get cataracts, increased pressure in the eye (glaucoma), and possible damage to the optic nerve if treated with Acthar Gel for a long time.
-
Allergic reactions to Acthar Gel. Your child may have an allergic reaction to Acthar Gel. Allergic reactions may not happen until your child has received several injections of Acthar Gel. Tell your doctor right away if your child has any of the following signs of an allergic reaction:
- skin rash
- swelling of the face, tongue, lips, or throat
- trouble breathing
- Changes in growth and physical development. Acthar Gel may affect your child's growth and physical development and may weaken his or her bones. This is more likely to happen with long term use of Acthar Gel.
- Enlarged heart. Acthar Gel may cause an increase in the size of your child's heart. This is more likely to happen with long term use of Acthar Gel but usually goes away after Acthar Gel is stopped.
The most common side effects of Acthar Gel for patients with infantile spasms include:
- Infections
- muscle contractions that you cannot control (convulsions)
- increased blood pressure
- irritability and changes in behavior
- fever
These are not all the possible side effects of Acthar Gel. Tell your doctor if your child has any side effect that bothers them or does not go away. For more information, ask your child's doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Acthar Gel?
- Store vials of Acthar Gel in the refrigerator between 36°F to 46°F (2°C to 8°C).
Throw away any vials after the expiration date printed on the label.
Keep Acthar Gel and all other medicines out of the reach of children
General information About Acthar Gel
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Acthar Gel for a condition for which it has not been prescribed. Do not give Acthar Gel to other people, even if they have the same symptoms. It may harm them.
This Medication Guide summarizes the most important information about Acthar Gel. If you would like more information, talk with your child's doctor. You can ask your child's doctor or pharmacist for information about Acthar Gel that is written for healthcare professionals. For more information, go to www.acthar.com or call 1-800-844-2830.
What are the ingredients in Acthar Gel?
Active ingredient: Corticotropin
Inactive ingredients: gelatin, phenol, cysteine, sodium hydroxide and/or acetic acid to adjust pH, and water for injectionManufactured for:
Mallinckrodt ARD LLC
Bridgewater, NJ 08807PL122
Rev 02/2024This Medication Guide has been approved by the U.S. Food and Drug Administration.
Mallinckrodt, the "M" brand mark, the Mallinckrodt Pharmaceuticals logo and other brands are trademarks of a Mallinckrodt company. © 2024 Mallinckrodt.
For a list of patents, see https://www.mallinckrodt.com/patents/
Mallinckrodt™
Pharmaceuticals -
INSTRUCTIONS FOR USE
ACTHAR® GEL [AK-thar jel]
(repository corticotropin injection)
for subcutaneous use only
Single-Dose Pre-filled SelfJect™ Injector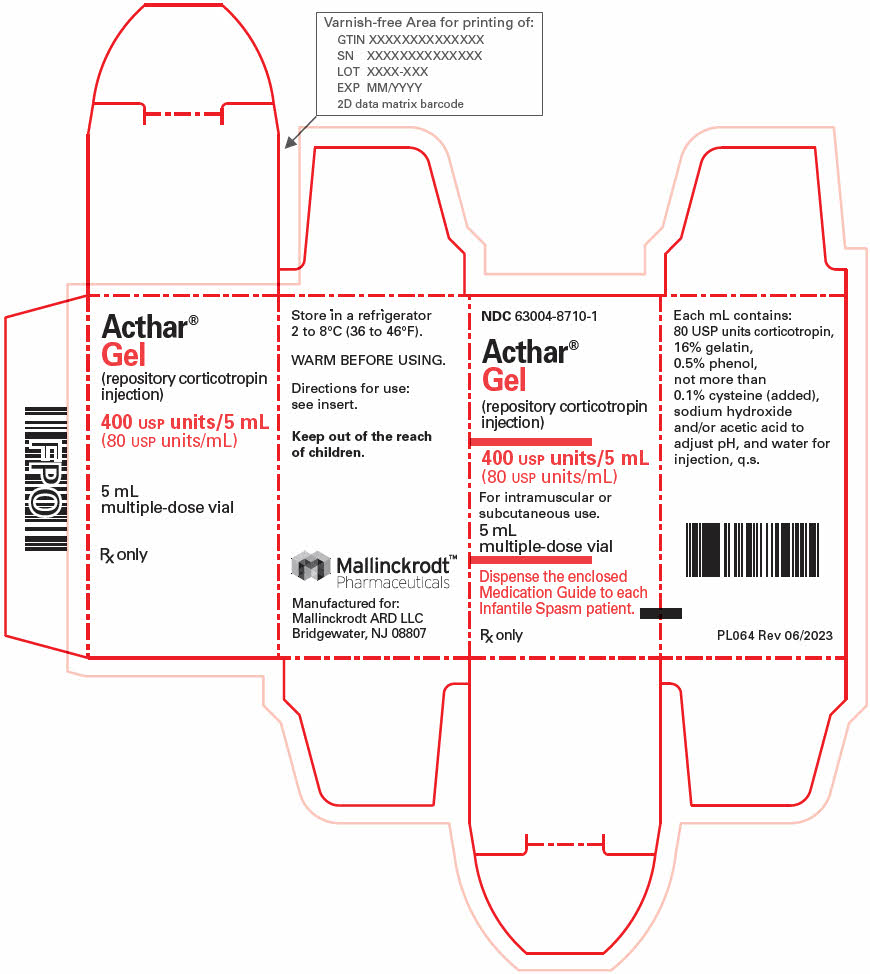
Green Body Injector: 40 USP Units/0.5 mL of Acthar Gel Step 1: Read These Instructions
- Carefully read, understand, and follow this Instructions for Use before you use the Acthar Gel single-dose pre-filled SelfJect injector.
- Contact your healthcare provider if you are unsure of how to give the injection.
Important Information - The Acthar Gel single-dose pre-filled SelfJect injector is for under the skin (subcutaneous) injection only.
- The Acthar Gel single-dose pre-filled SelfJect injector must only be given by adults 18 years of age and older.
- The Acthar Gel single-dose pre-filled SelfJect injector is a pre-filled injector for 1-time use only (single-dose).
- The Acthar Gel single-dose pre-filled SelfJect injector with the green body should only be used to give a single dose of 40 units. Doses other than 40 units cannot be given with this Acthar Gel single-dose pre-filled SelfJect injector.
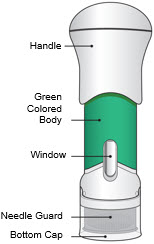
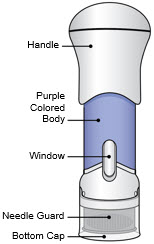
- This injector is not an auto-injector. The Handle must be pushed down by hand (see "The parts of the Acthar Gel single-dose pre-filled SelfJect injector" figure below).
- The injector has a Needle Guard to protect you and others from injury after it has been used.
The parts of the Acthar Gel single-dose pre-filled SelfJect injector are shown below: Before Use After the Injection is Complete 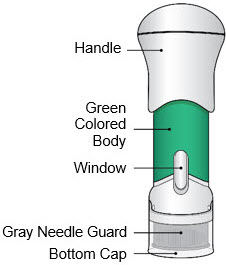
Storing Your Injector - Store the Acthar Gel single-dose pre-filled SelfJect injector in the refrigerator between 36°F and 46°F (2°C and 8°C).
- Store the Acthar Gel single-dose pre-filled SelfJect injector in the original plastic tray to protect the injector from light.
- When it is time to use the Acthar Gel single-dose pre-filled SelfJect injector, take 1 injector from the refrigerator and return any unused injectors to the refrigerator.
- After removing the injector from the refrigerator, it can be stored at room temperature between 68°F and 77°F (20°C and 25°C) for up to 24 hours.
- If the Acthar Gel single-dose pre-filled SelfJect injector has been left at room temperature for more than 24 hours, contact Mallinckrodt: 1-800-844-2830.
- Do not heat the Acthar Gel single-dose pre-filled SelfJect injector.
- Do not freeze the Acthar Gel single-dose pre-filled SelfJect injector.
- Do not put the Acthar Gel single-dose pre-filled SelfJect injector into direct sunlight.
- Keep the Acthar Gel single-dose pre-filled SelfJect injector and all medicines out of the reach of children.
Step 2: Prepare to Use the Acthar Gel single-dose pre-filled SelfJect injector - Choose a clean, well-lit room to give the injection.
- Take the injector tray out of the refrigerator.
- If the injector trays are in a multipack carton, take the injector tray out and return any unused injector trays to the refrigerator.
- Do not inject Acthar Gel right after removing it from the refrigerator. The injector does not work as well when cold.

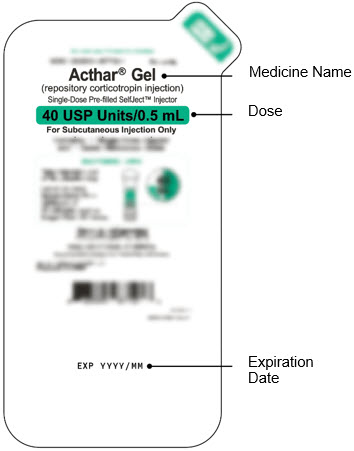
- Check that you have the correct medicine and dose.
- Check the expiration date (EXP) on the package.
Do not use if the expiration date has passed.
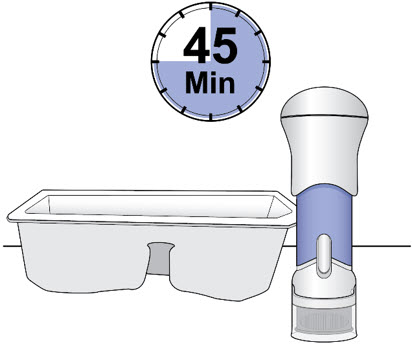
- Remove the injector from the injector tray and let it sit on a clean, dry, flat surface at room temperature to warm for a minimum of 45 minutes and no more than 24 hours before use.
Do not heat the injector.
- Before use, the injector should look like this.
- The Bottom Cap should remain on the injector until you give the injection.
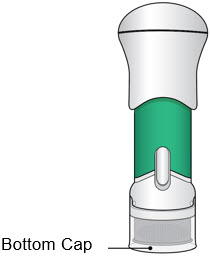
- Do not use the injector if the Bottom Cap is missing or not securely attached.
- If dropped, inspect the injector. Do not use if the injector is damaged or the Bottom Cap has come off.
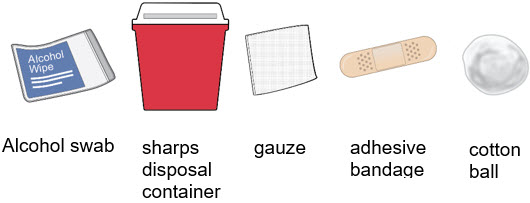
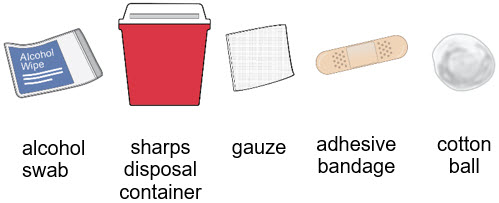
- Gather an alcohol swab and sharps disposal container.
- You may also gather an adhesive bandage, gauze, or cotton ball to use after the injection to stop any bleeding.
Step 3: Choose and Clean the Injection Site
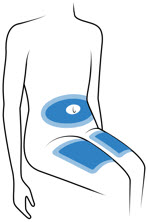
- The Acthar Gel single-dose pre-filled SelfJect injector is for under the skin (subcutaneous) injection only.
-
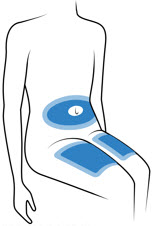
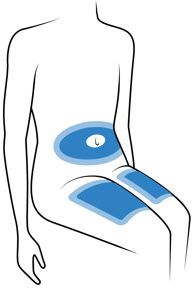
Choose 1 of these sites for injection:
- Upper thigh
- Belly (abdomen), not within 1-inch of belly button (navel)
- Back of arm (only if someone else is giving the injection)
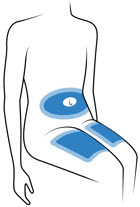
- Choose a different site for each injection. Do not use the same injection site more than 1 time each week.
- Do not inject through clothing.
-
Avoid injecting into:
- Irritated skin (red, swollen, or painful)
- Tattoos
- Warts
- Scars
- Birthmarks
- Within 1-inch of the knee
- Within 1-inch of the groin area
- Stretch marks
- Wash your hands well with soap and water.
- Clean the injection site with an alcohol swab.
-
Do not touch, fan, or blow on the injection site after you have cleaned it.
Step 4: Inspect and Uncap
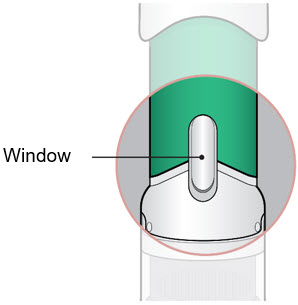
- Make sure the injector has warmed at room temperature for a minimum of 45 minutes before you continue.
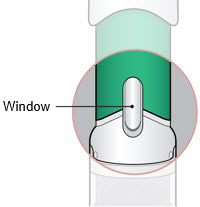
- Inspect the medicine in the Window.
- If needed, wipe away any condensation (water collected) from the Window with a dry cloth.
- Check the medicine in the Window. It should be a clear light yellow or orange solution.
- Do not use if the medicine is cloudy or you see particles.
- Note: It is normal to see air bubbles.
- Pull the Bottom Cap off to remove it and throw it away in your household trash.
- Avoid touching the Gray Needle Guard. The injector is now ready for use.
- After the injector has been warmed for a minimum of 45 minutes and you have prepared the injection site, use the injector right away.
Do not recap the injector as this may damage the needle.
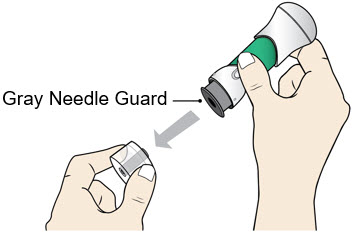
- When the Bottom Cap is removed, do not let the Gray Needle Guard touch any surface before injection.
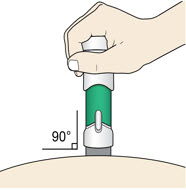
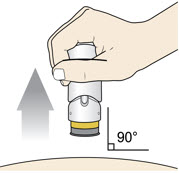
Step 5: Inject - Place the injector straight on the cleaned skin at a 90-degree angle.
Do not pinch the skin.
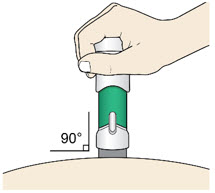
-
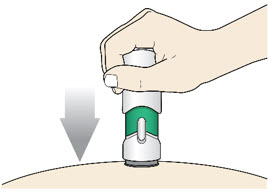
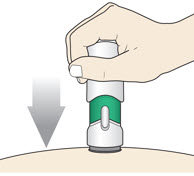
Slowly push the Handle down to inject the medicine.
Do not lift the injector during the injection. If you do, the injector will lock out and you will not get the full dose of medicine.
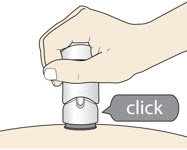
You may hear a "click" when the injection begins.
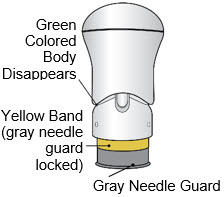
- The injection is complete when the Green Colored Body disappears and you may hear a "click".
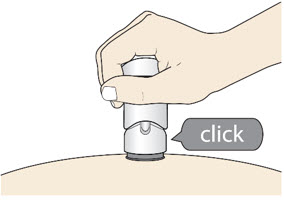
Before you lift the injector up, check to make sure that no part of the Green Colored Body remains visible. If you see color, continue pushing down on the Handle until the Green Colored Body disappears completely. Step 6: Lift - Lift the injector straight up at a 90-degree angle off the injection site.
If the Yellow Band is visible, it means that the Needle Guard is locked. You cannot use the injector again.
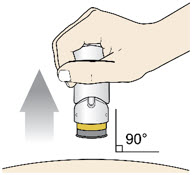
After the injection is complete, the injector should look like this: 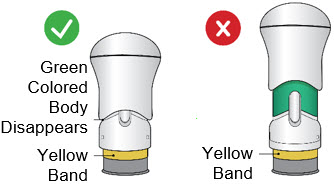
After use, the injector should not look like this: 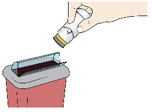
If you see the Green Colored Body and the Yellow Band at the same time, then you did not give the full dose.
Do not try to inject with this injector again.
Do not use another injector. Contact your healthcare provider.- If there is any bleeding at the injection site, cover the site with an adhesive bandage, a cotton ball, or a gauze. Apply gentle pressure until the bleeding stops.
Step 7: Throw Away (Dispose of) your Used Acthar Gel single-dose pre-filled SelfJect injector - Put your used Acthar Gel single-dose pre-filled SelfJect injector in an FDA-cleared sharps disposal container right away after use.
- Do not throw away your injector in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labelled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
- Do not recycle your used sharps disposal container.
Manufactured for:
Mallinckrodt ARD LLC
Bridgewater, NJ 08807SPC-0204 Rev 8
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 08/2024
-
Quick Reference Guide
ACTHAR® GEL [AK-thar jel]
(repository corticotropin injection)
for subcutaneous use only
40 USP Units/0.5 mL
Single-Dose Pre-filled SelfJect™ InjectorBefore use After the Injection is Complete 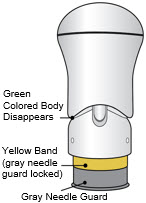

Important Information
- The Acthar Gel single-dose pre-filled SelfJect injector is for under the skin (subcutaneous) injection only.
1. Read These Instructions
- Contact your healthcare provider if you are unsure of how to give the injection.
- This is not an auto-injector. The Handle must be pushed down by hand.
- This is a single-dose injector (for 1-time use only).
- The injector has a Needle Guard to protect you after use.
2. Prepare to Use the Acthar Gel single-dose pre-filled SelfJect Injector
- Take the injector out of the refrigerator and remove it from its injector tray to warm at room temperature for a minimum of 45 minutes.
- Do not use the injector right after removing from the refrigerator. The injector does not work as well when cold. Do not heat.
- Check the expiration date (EXP) on the package.
- Complete this injection in a clean, well-lit room.
- Gather alcohol swab, adhesive bandage, and a sharps disposal container.
3. Choose and Clean the Injection Site
- For under the skin (subcutaneous) injection only.
-
Choose 1 of these sites for injection:
- -
- Upper thigh
- -
- Belly (abdomen), not within 1-inch of belly button (navel)
- -
- Back of arm (only if someone else is giving the injection)
- Choose a different site for each injection.
- Do not inject through clothing.
-
Avoid injecting into:
- -
- Irritated skin (red, swollen, or painful)
- -
- Tattoos, warts, scars, or birthmarks
- -
- Within 1-inch of knee or groin area
- -
- Stretch marks
- Wash your hands well with soap and water.
- Clean the injection site with an alcohol swab.
4. Inspect and Uncap
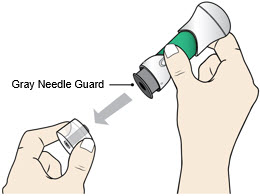
- Inspect the medicine in the Window. The medicine should be a clear light yellow or orange solution.
- Do not use if the medicine is cloudy or you see particles.
- It is normal to see air bubbles.
Pull Bottom Cap off to remove it and throw it away.
- Do not recap the injector as this may damage the needle.
- Avoid touching the Gray Needle Guard.
- Use the injector right away.
5. Inject
- (a)
-
Place the injector straight on the cleaned skin at a 90-degree angle.
Do not pinch the skin.
- (b)
-
Slowly push down the Handle to inject the medicine.
Do not lift the injector during the injection. You may hear a "click" when the injection begins.
- (c)
- The injection is complete when the Green Colored Body disappears and you may hear a "click."
6. Lift and Throw Away (Dispose of)
Lift the injector straight up at a 90-degree angle off the injection site.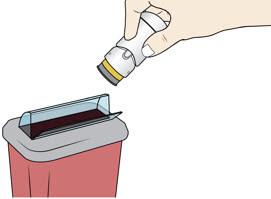
The Needle Guard is locked when you see the yellow band.
Place the injector in an approved sharps disposal container according to local regulations.
Do not throw away (dispose of) in your household trash.
SPC-0131 Rev 9 -
INSTRUCTIONS FOR USE
ACTHAR® GEL [AK-thar jel]
(repository corticotropin injection)
for subcutaneous use only
Single-Dose Pre-filled SelfJect™ Injector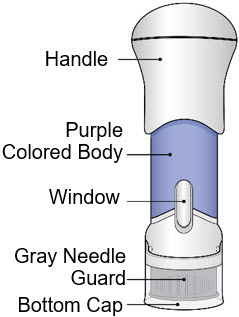
Purple Body Injector: 80 USP Units/mL of Acthar Gel Step 1: Read These Instructions
- Carefully read, understand, and follow this Instructions for Use before you use the Acthar Gel single-dose pre-filled SelfJect injector.
- Contact your healthcare provider if you are unsure of how to give the injection.
Important Information - The Acthar Gel single-dose pre-filled SelfJect injector is for under the skin (subcutaneous) injection only.
- The Acthar Gel single-dose pre-filled SelfJect injector must only be given by adults 18 years of age and older.
- The Acthar Gel single-dose pre-filled SelfJect injector is a pre-filled injector for 1-time use only (single-dose).
- The Acthar Gel single-dose pre-filled SelfJect injector with the purple body should only be used to give a single dose of 80 units. Doses other than 80 units cannot be given with this Acthar Gel single-dose pre-filled SelfJect injector.
- This injector is not an auto-injector. The Handle must be pushed down by hand (see "The parts of the Acthar Gel single-dose pre-filled SelfJect injector" figure below).
- The injector has a Needle Guard to protect you and others from injury after it has been used.
The parts of the Acthar Gel single-dose pre-filled SelfJect injector are shown below: Before Use After the Injection is Complete 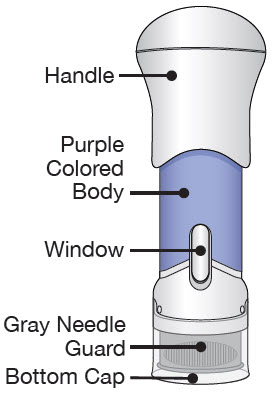
Storing Your Injector - Store the Acthar Gel single-dose pre-filled SelfJect injector in the refrigerator between 36°F and 46°F (2°C and 8°C).
- Store the Acthar Gel single-dose pre-filled SelfJect injector in the original plastic tray to protect the injector from light.
- When it is time to use the Acthar Gel single-dose pre-filled SelfJect injector, take 1 injector from the refrigerator and return any unused injectors to the refrigerator.
- After removing the injector from the refrigerator, it can be stored at room temperature between 68°F and 77°F (20°C and 25°C) for up to 24 hours.
- If the Acthar Gel single-dose pre-filled SelfJect injector has been left at room temperature for more than 24 hours, contact Mallinckrodt: 1-800-844-2830.
- Do not heat the Acthar Gel single-dose pre-filled SelfJect injector.
- Do not freeze the Acthar Gel single-dose pre-filled SelfJect injector.
- Do not put the Acthar Gel single-dose pre-filled SelfJect injector into direct sunlight.
- Keep the Acthar Gel single-dose pre-filled SelfJect injector and all medicines out of the reach of children.
Step 2: Prepare to Use the Acthar Gel single-dose pre-filled SelfJect injector - Choose a clean, well-lit room to give the injection.
- Take the injector tray out of the refrigerator.
- If the injector trays are in a multipack carton, take the injector tray out and return any unused injector trays to the refrigerator.
-
Do not inject Acthar Gel right after removing it from the refrigerator. The injector does not work as well when cold.

- Check that you have the correct medicine and dose.
- Check the expiration date (EXP) on the package.
Do not use if the expiration date has passed.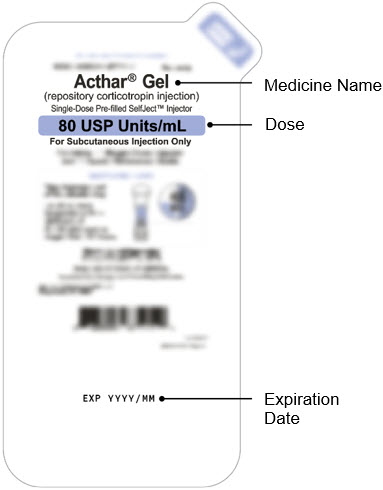
- Remove the injector from the injector tray and let it sit on a clean, dry, flat surface at room temperature to warm for a minimum of 45 minutes and no more than 24 hours before use.
Do not heat the injector.
- Before use, the injector should look like this.
- The Bottom Cap should remain on the injector until you give the injection.
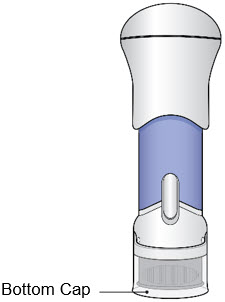
- Do not use the injector if the Bottom Cap is missing or not securely attached.
- If dropped, inspect the injector. Do not use if the injector is damaged or the Bottom Cap has come off.
- Gather an alcohol swab and sharps disposal container.
- You may also gather an adhesive bandage, gauze, or cotton ball to use after the injection to stop any bleeding.
Step 3: Choose and Clean the Injection Site - The Acthar Gel single-dose pre-filled SelfJect injector is for under the skin (subcutaneous) injection only.
-
Choose 1 of these sites for injection:
- Upper thigh
- Belly (abdomen), not within 1-inch of belly button (navel)
- Back of arm (only if someone else is giving the injection)
- Choose a different site for each injection. Do not use the same injection site more than 1 time each week.
- Do not inject through clothing.
-
Avoid injecting into:
- Irritated skin (red, swollen, or painful)
- Tattoos
- Warts
- Scars
- Birthmarks
- Within 1-inch of the knee
- Within 1-inch of the groin area
- Stretch marks
- Wash your hands well with soap and water.
- Clean the injection site with an alcohol swab.
- Do not touch, fan, or blow on the injection site after you have cleaned it.
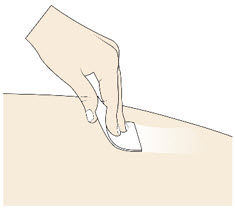
Step 4: Inspect and Uncap
- Make sure the injector has warmed at room temperature for a minimum of 45 minutes before you continue.
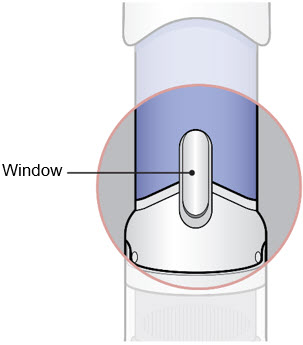
- Inspect the medicine in the Window.
- If needed, wipe away any condensation (water collected) from the Window with a dry cloth.
- Check the medicine in the Window. It should be a clear light yellow or orange solution.
- Do not use if the medicine is cloudy or you see particles.
- Note: It is normal to see air bubbles.
- Pull the Bottom Cap off to remove it and throw it away in your household trash.
- Avoid touching the Gray Needle Guard. The injector is now ready for use.
- After the injector has been warmed for a minimum of 45 minutes and you have prepared the injection site, use the injector right away.
Do not recap the injector as this may damage the needle.
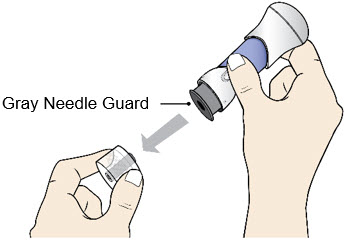
- When the Bottom Cap is removed, do not let the Gray Needle Guard touch any surface before injection.
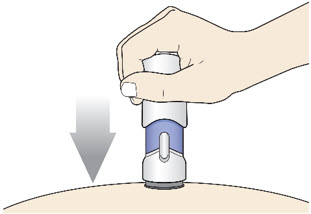
Step 5: Inject - Place the injector straight on the cleaned skin at a 90-degree angle.
Do not pinch the skin.
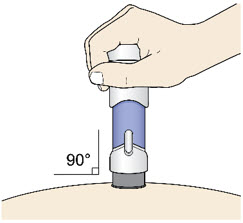
-
Slowly push the Handle down to inject the medicine.
Do not lift the injector during the injection. If you do, the injector will lock out and you will not get the full dose of medicine.
You may hear a "click" when the injection begins.
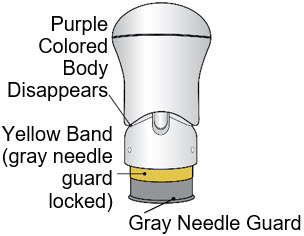
- The injection is complete when the Purple Colored Body disappears and you may hear a "click".
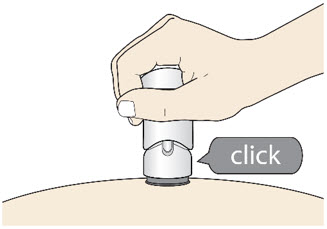
Before you lift the injector up, check to make sure that no part of the Purple Colored Body remains visible. If you see color, continue pushing down on the Handle until the Purple Colored Body disappears completely. Step 6: Lift - Lift the injector straight up at a 90-degree angle off the injection site.
If the Yellow Band is visible, it means that the Needle Guard is locked. You cannot use the injector again.
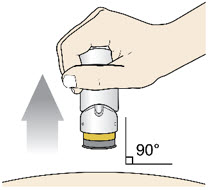
After the injection is complete, the injector should look like this: 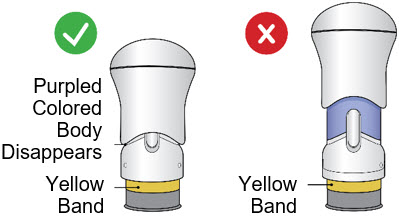
After use, the injector should not look like this: 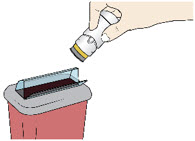
If you see the Purple Colored Body and the Yellow Band at the same time, then you did not give the full dose.
Do not try to inject with this injector again.
Do not use another injector. Contact your healthcare provider.- If there is any bleeding at the injection site, cover the site with an adhesive bandage, a cotton ball, or a gauze. Apply gentle pressure until the bleeding stops.
Step 7: Throw Away (Dispose of) your Used Acthar Gel single-dose pre-filled SelfJect injector - Put your used Acthar Gel single-dose pre-filled SelfJect injector in an FDA-cleared sharps disposal container right away after use.
- Do not throw away your injector in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labelled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
- Do not recycle your used sharps disposal container.
Manufactured for:
Mallinckrodt ARD LLC
Bridgewater, NJ 08807SPC-0205 Rev 8
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 08/2024
-
Quick Reference Guide
ACTHAR® GEL [AK-thar jel]
(repository corticotropin injection)
for subcutaneous use only
80 USP Units/mL
Single-Dose Pre-filled SelfJect™ InjectorBefore use After the Injection is Complete 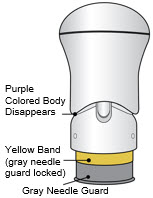

Important Information
- The Acthar Gel single-dose pre-filled SelfJect injector is for under the skin (subcutaneous) injection only.
1. Read These Instructions
- Contact your healthcare provider if you are unsure of how to give the injection.
- This is not an auto-injector. The Handle must be pushed down by hand.
- This is a single-dose injector (for 1-time use only).
- The injector has a Needle Guard to protect you after use.
2. Prepare to Use the Acthar Gel single-dose pre-filled SelfJect Injector
- Take the injector out of the refrigerator and remove it from its injector tray to warm at room temperature for a minimum of 45 minutes.
- Do not use the injector right after removing from the refrigerator. The injector does not work as well when cold. Do not heat.
- Check the expiration date (EXP) on the package.
- Complete this injection in a clean, well-lit room.
- Gather alcohol swab, adhesive bandage, and a sharps disposal container.
3. Choose and Clean the Injection Site
- For under the skin (subcutaneous) injection only.
-
Choose 1 of these sites for injection:
- -
- Upper thigh
- -
- Belly (abdomen), not within 1-inch of belly button (navel)
- -
- Back of arm (only if someone else is giving the injection)
- Choose a different site for each injection.
- Do not inject through clothing.
-
Avoid injecting into:
- -
- Irritated skin (red, swollen, or painful)
- -
- Tattoos, warts, scars, or birthmarks
- -
- Within 1-inch of knee or groin area
- -
- Stretch marks
- Wash your hands well with soap and water.
- Clean the injection site with an alcohol swab.
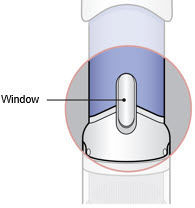
4. Inspect and Uncap
- Inspect the medicine in the Window. The medicine should be a clear light yellow or orange solution.
- Do not use if the medicine is cloudy or you see particles.
- It is normal to see air bubbles.
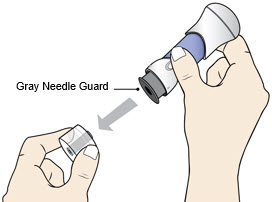
Pull Bottom Cap off to remove it and throw it away.
- Do not recap the injector as this may damage the needle.
- Avoid touching the Gray Needle Guard.
- Use the injector right away.
5. Inject
- (a)
-
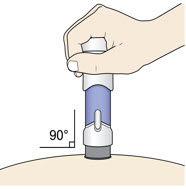
Place the injector straight on the cleaned skin at a 90-degree angle.
Do not pinch the skin.
- (b)
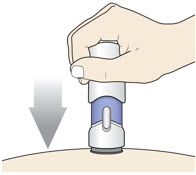
-
Slowly push down the Handle to inject the medicine.
Do not lift the injector during the injection. You may hear a "click" when the injection begins.
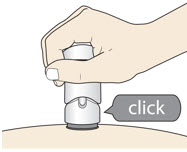
- (c)
- The injection is complete when the Purple Colored Body disappears and you may hear a "click".
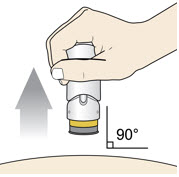
6. Lift and Throw Away (Dispose of)
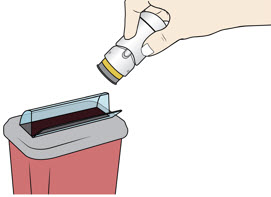 Lift the injector straight up at a 90-degree angle off the injection site.
Lift the injector straight up at a 90-degree angle off the injection site.
The Needle Guard is locked when you see the yellow band. Place the injector in an approved sharps disposal container according to local regulations.
Place the injector in an approved sharps disposal container according to local regulations.
Do not throw away (dispose of) in your household trash.
SPC-0132 Rev 9 - PRINCIPAL DISPLAY PANEL - 5 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 4 x 0.5 mL Injector Carton
- PRINCIPAL DISPLAY PANEL - 4 x 1.0 mL Injector Carton
-
INGREDIENTS AND APPEARANCE
ACTHAR
repository corticotropin injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63004-8710 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CORTICOTROPIN (UNII: K0U68Q2TXA) (CORTICOTROPIN - UNII:K0U68Q2TXA) CORTICOTROPIN 80 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) PHENOL (UNII: 339NCG44TV) CYSTEINE (UNII: K848JZ4886) SODIUM HYDROXIDE (UNII: 55X04QC32I) ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63004-8710-1 1 in 1 CARTON 01/07/2013 1 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 2 NDC:63004-8710-2 1 in 1 CARTON 01/07/2013 2 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA008372 01/07/2013 ACTHAR
repository corticotropin injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63004-8712 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CORTICOTROPIN (UNII: K0U68Q2TXA) (CORTICOTROPIN - UNII:K0U68Q2TXA) CORTICOTROPIN 40 [USP'U] in 0.5 mL Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) PHENOL (UNII: 339NCG44TV) CYSTEINE (UNII: K848JZ4886) SODIUM HYDROXIDE (UNII: 55X04QC32I) ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63004-8712-4 4 in 1 CARTON 02/29/2024 1 NDC:63004-8712-1 1 in 1 TRAY 1 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA008372 02/29/2024 ACTHAR
repository corticotropin injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63004-8711 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CORTICOTROPIN (UNII: K0U68Q2TXA) (CORTICOTROPIN - UNII:K0U68Q2TXA) CORTICOTROPIN 80 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) PHENOL (UNII: 339NCG44TV) CYSTEINE (UNII: K848JZ4886) SODIUM HYDROXIDE (UNII: 55X04QC32I) ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63004-8711-4 4 in 1 CARTON 02/29/2024 1 NDC:63004-8711-1 1 in 1 TRAY 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA008372 02/29/2024 Labeler - Mallinckrodt ARD LLC (625130828)






