Label: OLOPATADINE HYDROCHLORIDE solution/ drops
- NDC Code(s): 62332-501-05
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 21, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Olopatadine hydrochloride ophthalmic solution USP, 0.1% is a sterile ophthalmic solution containing olopatadine, a relatively selective H1-receptor antagonist and inhibitor of histamine release from the mast cell for topical administration to the eyes. Olopatadine hydrochloride, USP is a white, crystalline, water-soluble powder with a molecular weight of 373.88. The chemical structure is presented below:
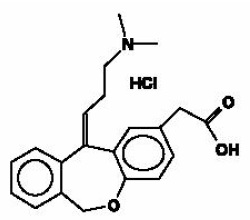
Chemical Name: 11-[(Z)-3-(Dimethylamino)propylidene]-6-11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride
Each mL of olopatadine hydrochloride ophthalmic solution USP, 0.1 % contains: Active: 1.11 mg olopatadine hydrochloride, USP equivalent to 1 mg olopatadine.
Preservative: Benzalkonium chloride 0.01%. Inactives: Dibasic sodium phosphate; Sodium chloride; Hydrochloric acid/Sodium hydroxide (adjust pH); and Water for Injection. It has a pH of approximately 7 and an osmolality of approximately 300 mOsm/kg.
-
CLINICAL PHARMACOLOGY
Olopatadine is an inhibitor of the release of histamine from the mast cell and a relatively selective histamine H1-antagonist that inhibits the in vivo and in vitro type 1 immediate hypersensitivity reaction including inhibition of histamine induced effects on human conjunctival epithelial cells. Olopatadine is devoid of effects on alpha-adrenergic, dopamine and muscarinic type 1 and 2 receptors. Following topical ocular administration in man, olopatadine was shown to have low systemic exposure. Two studies in normal volunteers (totaling 24 subjects) dosed bilaterally with olopatadine 0.15% ophthalmic solution once every 12 hours for 2 weeks demonstrated plasma concentrations to be generally below the quantitation limit of the assay (<0.5 ng/mL). Samples in which olopatadine was quantifiable were typically found within 2 hours of dosing and ranged from 0.5 to 1.3 ng/mL. The half-life in plasma was approximately 3 hours, and elimination was predominantly through renal excretion. Approximately 60-70% of the dose was recovered in the urine as parent drug. Two metabolites, the mono-desmethyl and the N-oxide, were detected at low concentrations in the urine.
Results from an environmental study demonstrated that olopatadine hydrochloride ophthalmic solution USP, 0.1% was effective in the treatment of the signs and symptoms of allergic conjunctivitis when dosed twice daily for up to 6 weeks. Results from conjunctival antigen challenge studies demonstrated that Olopatadine hydrochloride ophthalmic solution USP, 0.1% when subjects were challenged with antigen both initially and up to 8 hours after dosing, was significantly more effective than its vehicle in preventing ocular itching associated with allergic conjunctivitis. - INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
INFORMATION FOR PATIENTS
To prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
Patients should be advised not to wear a contact lens if their eye is red. Olopatadine hydrochloride ophthalmic solution USP, 0.1% should not be used to treat contact lens related irritation. The preservative in Olopatadine hydrochloride ophthalmic solution USP, 0.1%, benzalkonium chloride, may be absorbed by soft contact lenses. Patients who wear soft contact lenses and whose eyes are not red should be instructed to wait at least ten minutes after instilling Olopatadine hydrochloride ophthalmic solution USP, 0.1% before they insert their contact lenses.CARCINOGENESIS AND MUTAGENESIS AND IMPAIRMENT OF FERTILITY
Olopatadine administered orally was not carcinogenic in mice and rats in doses up to 500 mg/kg/day and 200 mg/kg/day, respectively. Based on a 40 μL drop size, these doses were 78,125 and 31,250 times higher than the maximum recommended ocular human dose (MROHD). No mutagenic potential was observed when olopatadine was tested in an in vitro bacterial reverse mutation (Ames) test, an in vitro mammalian chromosome aberration assay or an in vivo mouse micronucleus test. Olopatadine administered to male and female rats at oral doses of 62,500 times MROHD level resulted in a slight decrease in the fertility index and reduced implantation rate; no effects on reproductive function were observed at doses of 7,800 times the maximum recommended ocular human use level.
PREGNANCY
Pregnancy Category C. Olopatadine was found not to be teratogenic in rats and rabbits. However, rats treated at 600 mg/kg/day, or 93,750 times the MROHD and rabbits treated at 400 mg/kg/day, or 62,500 times the MROHD, during organogenesis showed a decrease in live fetuses. There are, however, no adequate and well controlled studies in pregnant women. Because animal studies are not always predictive of human responses, this drug should be used in pregnant women only if the potential benefit to the mother justifies the potential risk to the embryo or fetus.
NURSING MOTHERS
Olopatadine has been identified in the milk of nursing rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in the human breast milk. Nevertheless, caution should be exercised when Olopatadine hydrochloride ophthalmic solution USP, 0.1% is administered to a nursing mother.
-
ADVERSE REACTIONS
Headaches have been reported at an incidence of 7%. The following adverse experiences have been reported in less than 5% of patients: Asthenia, blurred vision, burning or stinging, cold syndrome, dry eye, foreign body sensation, hyperemia, hypersensitivity, keratitis, lid edema, nausea, pharyngitis, pruritis, rhinitis, sinusitis, and taste perversion. Some of these events were similar to the underlying disease being studied.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Olopatadine hydrochloride ophthalmic solution USP, 0.1% is supplied as follows: 5 mL in 5 mL screw neck white low density polyethylene bottle.
5 mL: NDC 62332-501-05
Storage: Store at 4° to 25°C (39° to 77°F)Rx Only
Manufactured for:
Alembic Pharmaceuticals, Inc.
750 Route 202, Bridgewater, NJ 08807
USA
Manufactured by:
Gland Pharma Limited
D.P. Pally, Dundigal Post,
Hyderabad-500 043, IndiaRevised: 12/2018
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OLOPATADINE HYDROCHLORIDE
olopatadine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62332-501 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLOPATADINE HYDROCHLORIDE (UNII: 2XG66W44KF) (OLOPATADINE - UNII:D27V6190PM) OLOPATADINE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62332-501-05 1 in 1 CARTON 12/07/2018 1 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209919 12/07/2018 Labeler - Alembic Pharmaceuticals Inc. (079288842) Establishment Name Address ID/FEI Business Operations Gland Pharma Limited 918601238 MANUFACTURE(62332-501)




