Label: MICONAZOLE 7- miconazole nitrate cream
- NDC Code(s): 49781-064-75
- Packager: Cardinal Health (Leader) 49781
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
-
Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back, or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
- Ask a doctor or pharmacist before use if you are
-
When using this product
- do not use tampons, douches, spermicides or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- do not have vaginal intercourse
- mild increase in vaginal burning, itching or irritation may occur
- if you do not get complete relief ask a doctor before using another product.
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- before using this product read the enclosed consumer information leaflet for complete directions and information
-
adults and children 12 years of age and over:
- applicator: insert 1 applicatorful into the vagina at bedtime for 7 nights in a row. Wash applicator after use.
- use the same tube of cream if you have itching and irritation on the skin outside the vagina. Squeeze a small amount of cream onto your fingertip. Apply to itchy, irritated skin outside the vagina (vulva). Use 2 times daily for up to 7 days as needed.
- children under 12 years of age: ask a doctor
- Other information
- Inactive ingredients
- Questions?
-
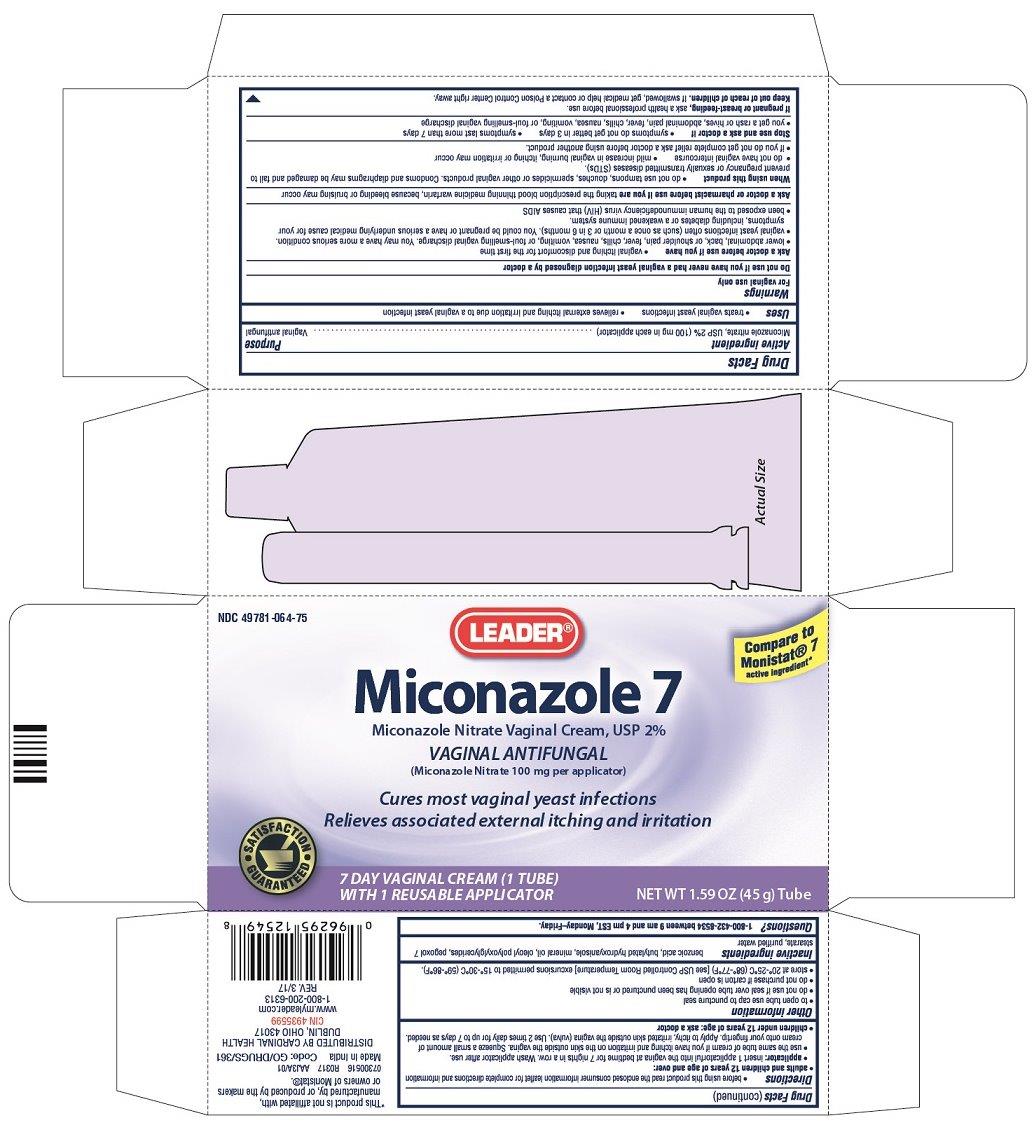
Principal display panel
NDC 49781-064-75
LEADER®
Compare to Monistat® 7
active ingredient*Miconazole 7
Miconazole Nitrate Vaginal Cream, USP 2%VAGINAL ANTIFUNGAL
(Miconazole Nitrate 100 mg per applicator)Cures most vaginal yeast infections
Relieves associated external itching and irritationSATISFACTION GUARANTEED
7 DAY VAGINAL CREAM (1 TUBE)
WITH 1 REUSABLE APPLICATORNET WT. 1.59 OZ (45 g) Tube
-
INGREDIENTS AND APPEARANCE
MICONAZOLE 7
miconazole nitrate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49781-064 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 20 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) MINERAL OIL (UNII: T5L8T28FGP) APRICOT KERNEL OIL PEG-6 ESTERS (UNII: DRG3KJZ1TJ) PEGOXOL 7 STEARATE (UNII: 3EW5AXE5X5) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49781-064-75 1 in 1 CARTON 05/31/2014 1 45 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074164 05/31/2014 Labeler - Cardinal Health (Leader) 49781 (097537435)
