Label: EBGLYSS- lebrikizumab-lbkz injection, solution
- NDC Code(s): 0002-7772-01, 0002-7772-11, 0002-7772-62, 0002-7797-01, view more
- Packager: Eli Lilly and Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EBGLYSS safely and effectively. See full prescribing information for EBGLYSS. EBGLYSS (lebrikizumab-lbkz), injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
EBGLYSS is indicated for the treatment of adults and pediatric patients 12 years of age and older who weigh at least 40 kg with moderate-to-severe atopic dermatitis whose disease is not ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Vaccination Prior to Administration of EBGLYSS - Complete all age-appropriate vaccinations according to current immunization guidelines [see Warnings and Precautions (5.4)]. 2.2 ...
-
3 DOSAGE FORMS AND STRENGTHS
EBGLYSS is a clear to opalescent, colorless to slightly yellow to slightly brown solution available as follows: Injection: 250 mg/2 mL in a single-dose prefilled pen - Injection: 250 mg/2 mL in a ...
-
4 CONTRAINDICATIONS
EBGLYSS is contraindicated in patients with prior serious hypersensitivity to lebrikizumab-lbkz or any excipients of EBGLYSS [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity - Hypersensitivity reactions, including angioedema and urticaria, have been reported with use of EBGLYSS. If a serious hypersensitivity reaction occurs, discontinue EBGLYSS ...
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling: Hypersensitivity [see Warnings and Precautions (5.1)] Conjunctivitis and Keratitis [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Available data on lebrikizumab-lbkz use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or other ...
-
10 OVERDOSAGE
In the event of overdosage, contact Poison Control (1-800-222-1222) for the latest recommendations and monitor the patient for any signs or symptoms of adverse reactions and institute appropriate ...
-
11 DESCRIPTION
Lebrikizumab-lbkz, an interleukin-13 antagonist, is an immunoglobulin G4 (IgG4) monoclonal antibody that binds to interleukin (IL)-13 and inhibits IL-13 signaling. Lebrikizumab-lbkz is produced in ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Lebrikizumab-lbkz is an IgG4 monoclonal antibody that binds with high affinity and slow off-rate to interleukin (IL)-13 and allows IL-13 to bind to IL-13Rα1 but ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of lebrikizumab-lbkz. No effects on ...
-
14 CLINICAL STUDIES
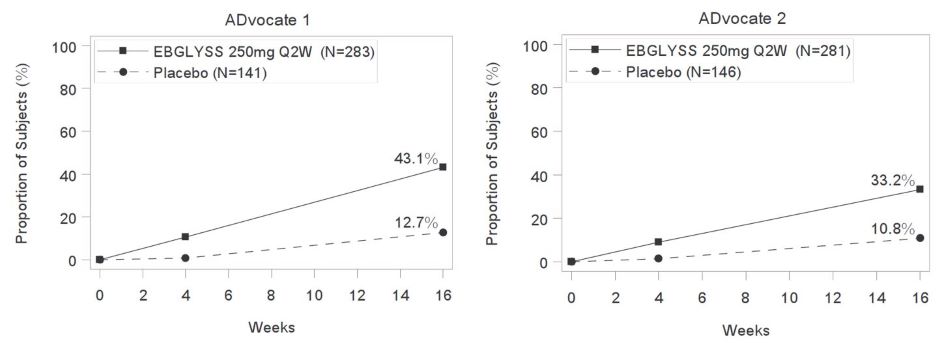
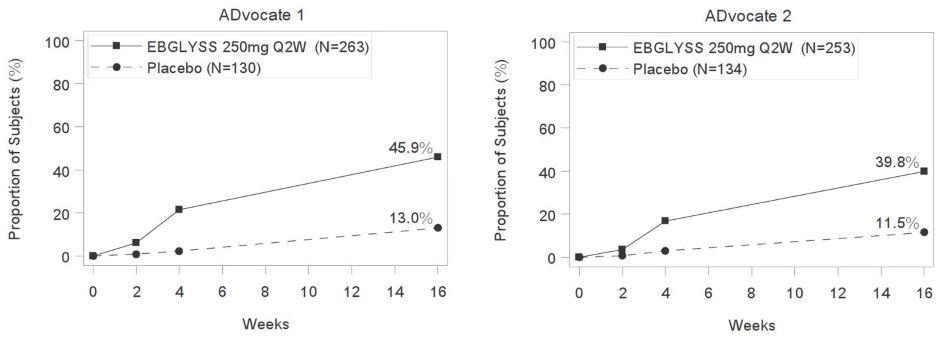
14.1 Atopic Dermatitis - Three multicenter, randomized, double-blind, placebo-controlled trials, ADvocate 1, ADvocate 2 and ADhere (NCT04146363, NCT04178967, NCT04250337) enrolled a total of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied - EBGLYSS (lebrikizumab-lbkz) injection is a sterile, preservative free, clear to opalescent, colorless to slightly yellow to slightly brown solution, available in a single-dose ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Administration Instructions: Provide guidance to patients and ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration - Revised: 09/2024 - EBG-0001-PPI-202409 - PATIENT INFORMATION - EBGLYSSTM ...
-
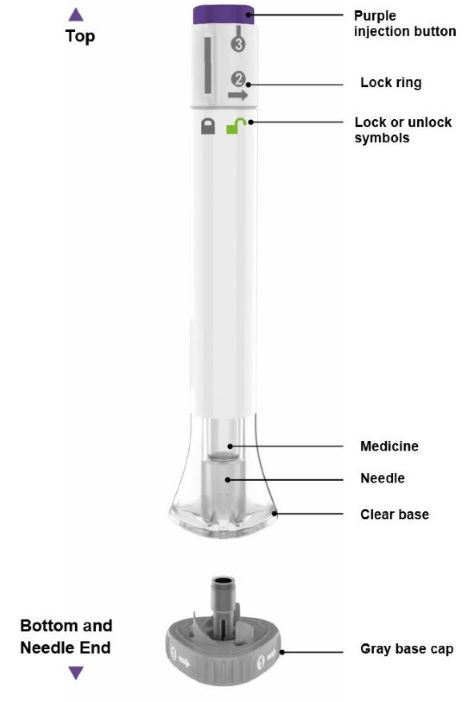
PREFILLED PEN INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE - EBGLYSS™ [EHB-glihs] (lebrikizumab-lbkz) injection, for subcutaneous use - Single-Dose Prefilled Pen - This Instructions for Use contains information on how to ...
-
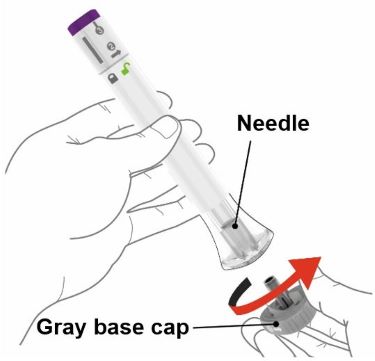
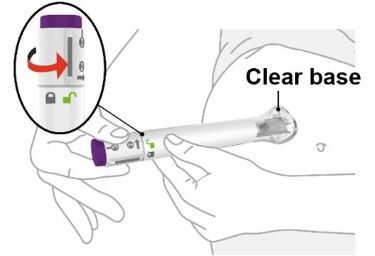
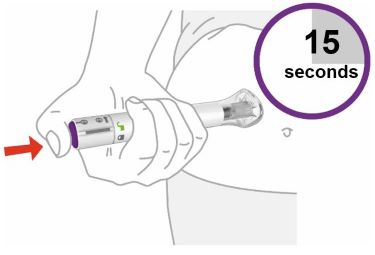
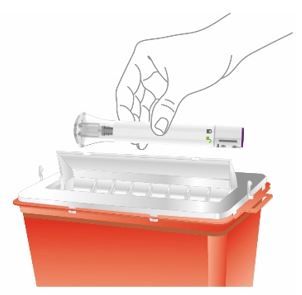
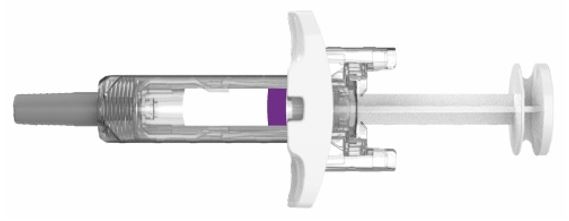
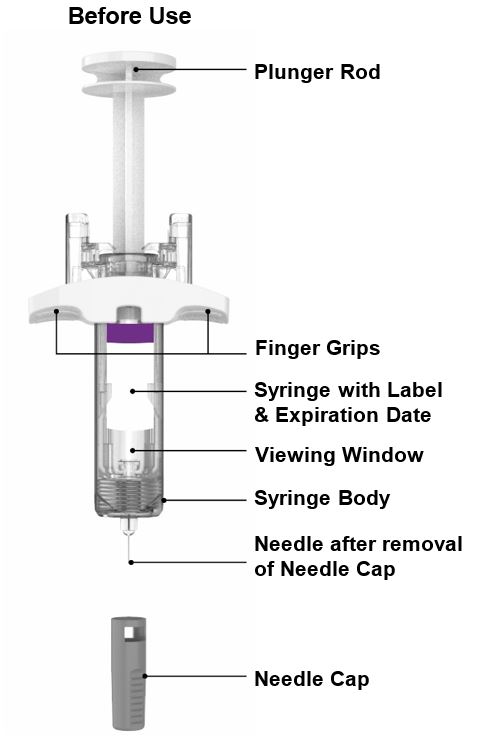
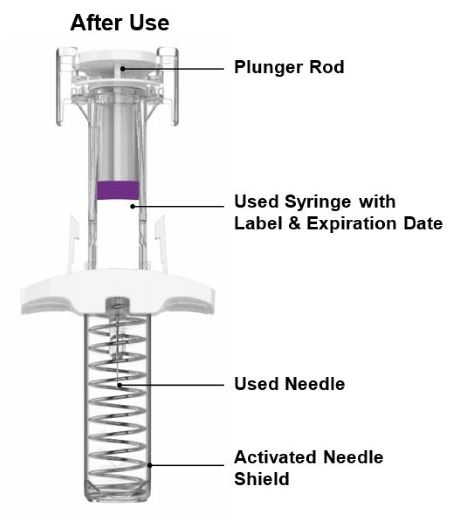
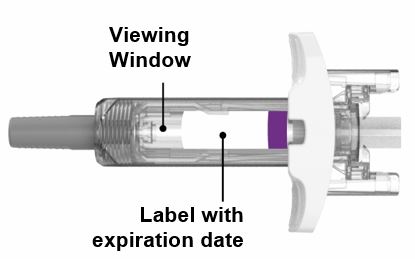
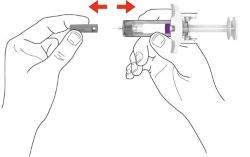
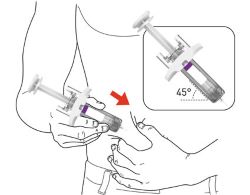
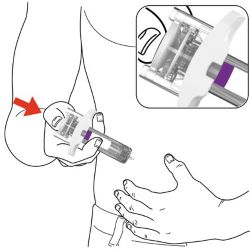
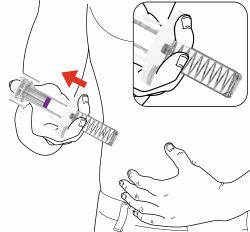
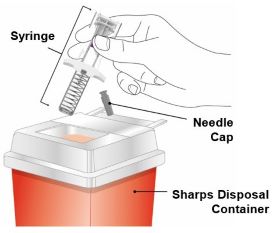
PREFILLED SYRINGE INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE - EBGLYSS™ [EHB-glihs] (lebrikizumab-lbkz) injection, for subcutaneous use - Single-Dose Prefilled Syringe with Needle Shield - This Instructions for Use contains ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – Ebglyss 250 mg Prefilled Pen - NDC 0002-7772-11 - Ebglyss TM - (lebrikizumab-lbkz) injection - 250 mg / 2 mL - 1 x 250 mg/2 mL - Single-Dose Prefilled Pen - For Subcutaneous Use ...
-
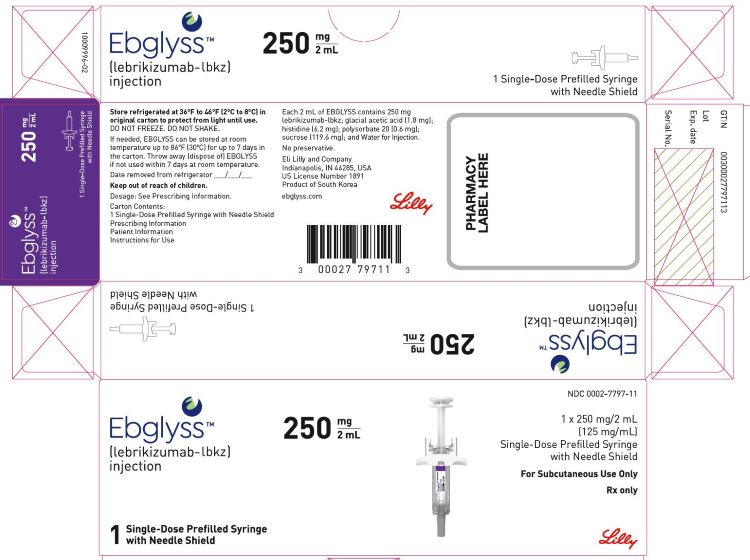
PRINCIPAL DISPLAY PANELPACKAGE LABEL – Ebglyss 250 mg Prefilled Syringe - NDC 0002-7797-11 - Ebglyss TM - (lebrikizumab-lbkz) injection - 250 mg / 2 mL - 1 x 250 mg/2 mL - (125 mg/mL) Single-Dose Prefilled Syringe with ...
-
INGREDIENTS AND APPEARANCEProduct Information