Label: STRATUSCARE BISACODYL- bisacodyl suppository
- NDC Code(s): 58980-415-04, 58980-415-08, 58980-415-12, 58980-415-16, view more
- Packager: Stratus Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
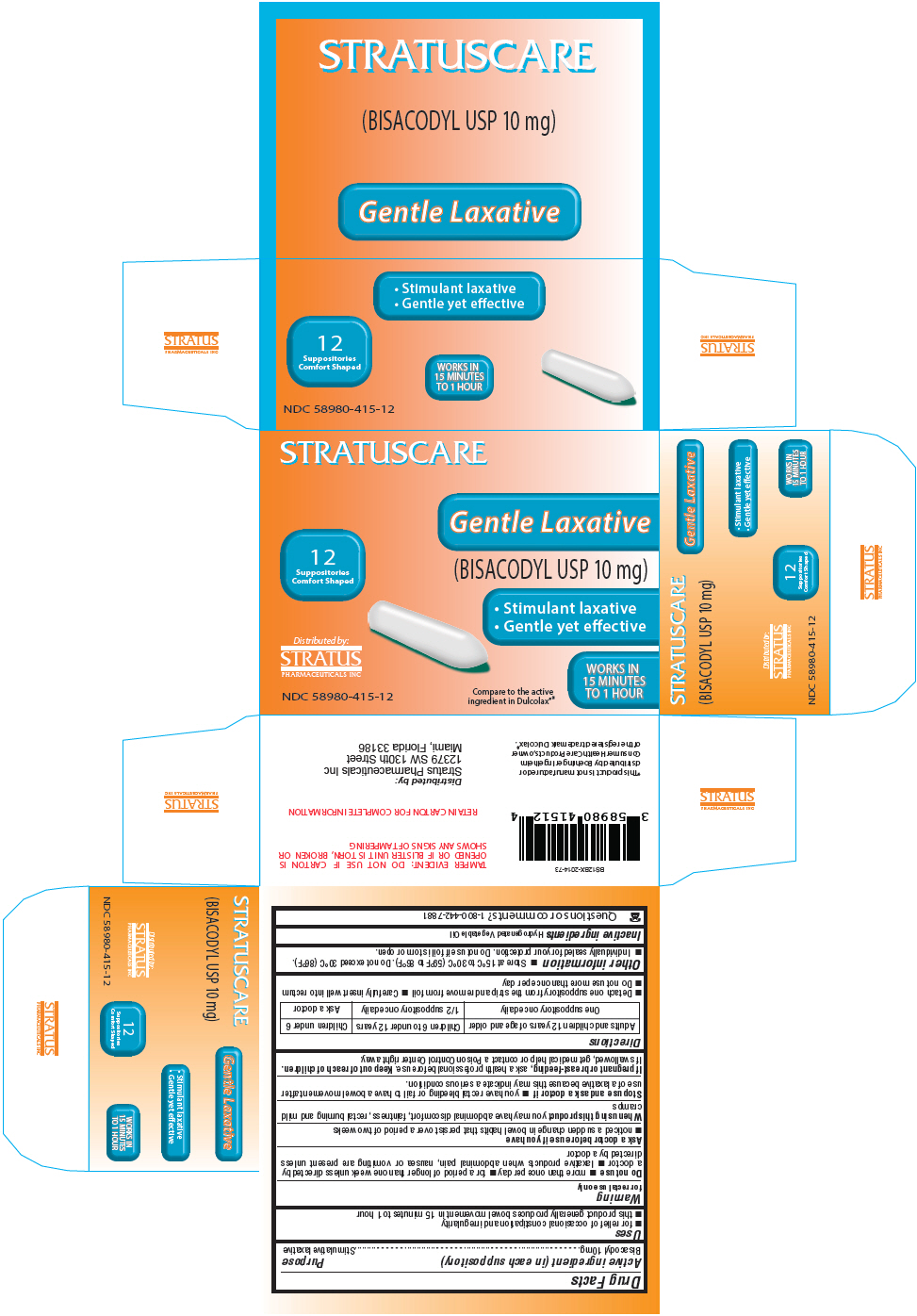
Active ingredient (in each suppository)Bisacodyl 10mg
-
PurposeStimulative laxative
-
Usesfor relief of occasional constipation and irregularity - this product generally produces bowel movement in 15 minutes to 1 hour
-
Warningfor rectal use only - Do not use - more than once per day - for a period of longer than one week unless directed by a doctor - laxative products when abdominal pain, nausea or vomiting are present ...
-
DirectionsAdults and children 12 years of age and olderChildren 6 to under 12 yearsChildren under 6 - One suppository once daily1/2 suppository once dailyAsk a doctor - Detach one suppository from ...
-
Other informationStore at 15°C to 30°C (59°F to 86°F). Do not exceed 30°C (86°F). Individually sealed for your protection. Do not use if foil is torn or open.
-
Inactive ingredientsHydrogenated Vegetable Oil
-
Questions or comments?1-800-442-7881
-
SPL UNCLASSIFIED SECTIONDistributed by: Stratus Pharmaceuticals Inc - 12379 SW 130th Street - Miami, Florida 33186
-
PRINCIPAL DISPLAY PANEL - 10 mg Blister Pack BoxSTRATUSCARE - 12 - Suppositories - Comfort Shaped - Distributed by: STRATUS - PHARMACEUTICALS INC - NDC 58980-415-12 - Gentle Laxative - (BISACODYL USP 10 mg) Stimulant laxative - Gentle yet effective - WORKS IN - 15 ...
-
INGREDIENTS AND APPEARANCEProduct Information