Label: WALGREENS GRAPE FLAVOR- children cough and chest congestion liquid
- NDC Code(s): 0363-7470-04
- Packager: WALGREENS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 20 mL)
- Purposes
- Uses
- Warnings
-
Do not use
If your are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- keep dosing cup with product
- •
- mL = milliliter
Age
Dose
children under 4 years
do not use
children 4 to under 6 years
5 mL every 4 hours
children 6 to under 12 years
10 mL every 4 hours
adults and children 12 years and older
20 mL every 4 hours
- Other information
- Inactive ingredients
- Questions or Comments
-
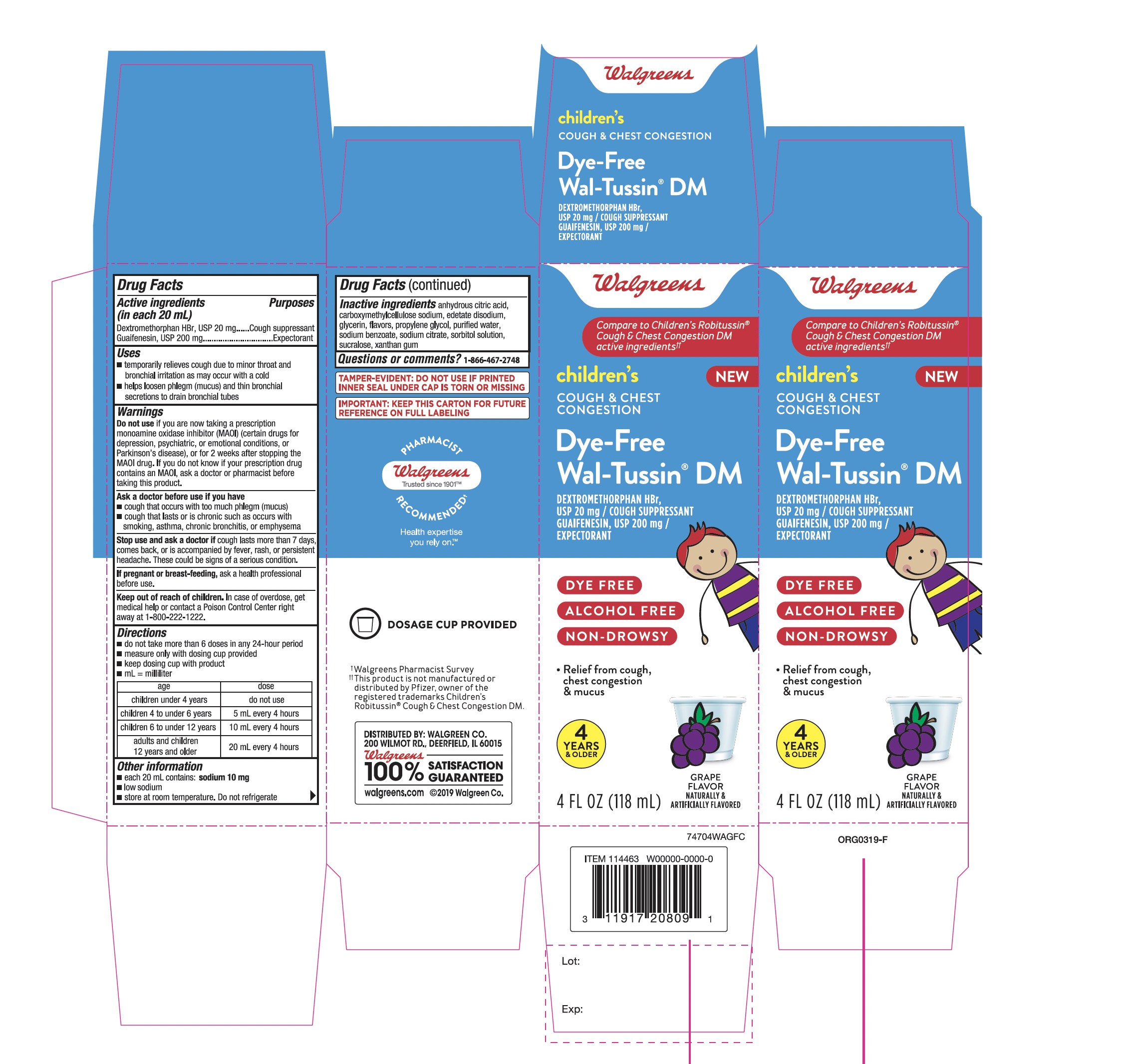
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 FL OZ (118 mL Bottle)
Walgreens
Compare to the Children's Robitussin® Cough & Chest Congestion DM active ingredients††
NDC 0363-7470-04
Children'sCOUGH& CHESTCONGESTION
Dye-FreeWal-Tussin® DM
DEXTROMETHORPHAN HBr,
USP 20 mg/COUGH SUPPRESSANT
GUAIFENESIN, USP 200 mg/EXPECTORANTDYE FREE
ALCOHOL FREE
NON-DROWSY
- •
- Relief from cough chest congestion & mucus
4 YEARS & OLDER
GRAPE FLAVORNATURALLY & ARTIFICIALLY FALVORED
4 FL OZ (118 mL)
TAMPER-EVIDENT: DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS TORN OR MISSING
IMPORTANT: KEEP THIS CARTON FOR FUTURE REFERENCE ON FULL LABELING
†Walgreens Pharmacist Survey
††This product is not manufactured or distributed by Pfizer, owner of the registered trademarks Children’s Robitussin® Cough & Chest Congestion DM.
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
Walgreens
100% SATISFACTION GUARANTEED
-
INGREDIENTS AND APPEARANCE
WALGREENS GRAPE FLAVOR
children cough and chest congestion liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-7470 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color PURPLE Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-7470-04 1 in 1 CARTON 05/15/2019 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/15/2019 Labeler - WALGREENS (008965063)