Label: FLUOCINOLONE ACETONIDE OIL oil
- NDC Code(s): 64980-329-20
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
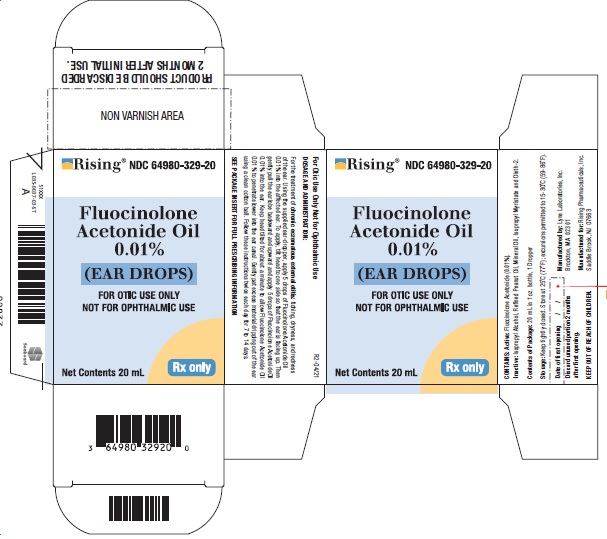
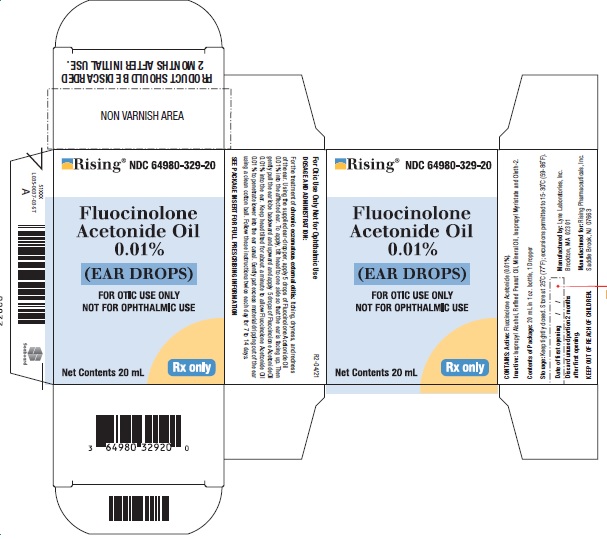
SPL UNCLASSIFIED SECTIONNDC 64980-329-20 - For Otic Use Only - Not for Ophthalmic Use
-
DESCRIPTIONFluocinolone Acetonide Oil 0.01% (Ear Drops) contains fluocinolone acetonide {(6α,11β,16α)-6,9-difluoro-11,21-dihydroxy- 16,17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione, cyclic ...
-
CLINICAL PHARMACOLOGYLike other topical corticosteroids, fluocinolone acetonide has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical ...
-
PHARMACOKINETICSPharmacokinetics: The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle and the integrity of the epidermal barrier. Occlusion of ...
-
CLINICAL STUDIESEfficacy in a placebo-controlled study for the treatment of chronic eczematous external otitis on 154 patients (adults and children 2 years of age and older) treated with five drops per ear of ...
-
INDICATION AND USAGEFluocinolone acetonide oil 0.01% is a low to medium potency corticosteroid indicated for the treatment of chronic eczematous external otitis in adults and pediatric patients 2 years and ...
-
CONTRAINDICATIONSFluocinolone acetonide oil 0.01% is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation. This product contains refined peanut oil NF ...
-
PRECAUTIONSGeneral: Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency ...
-
ADVERSE REACTIONSThe following local adverse reactions have been reported infrequently with topical corticosteroids. They may occur more frequently with the use of occlusive dressings, especially with higher ...
-
OVERDOSAGETopically applied fluocinolone acetonide oil 0.01% can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
-
DOSAGE AND ADMINISTRATIONFor the treatment of chronic eczematous external otitis, using the supplied ear-dropper, apply 5 drops of fluocinolone acetonide oil 0.01% into the affected ear. To apply, tilt head to one side so ...
-
HOW SUPPLIEDFluocinolone Acetonide Oil 0.01% (Ear Drops) is supplied in 1 fluid ounce bottles containing 20 mL (Dropper Included) (NDC # 64980-329-20). Keep tightly closed. Store at 20°-25°C (68°to 77°F) ...
-
PRINCIPAL DISPLAY PANEL———PRINCIPAL DISPLAY PANEL——— Rising® PHARMACEUTICALS - NDC 64980-329-20FluocinoloneAcetonide Oil0.01%(EAR DROPS) FOR OTIC USE ONLY - NOT FOR OPHTHALMIC USE - Net Contents 20 ...
-
INGREDIENTS AND APPEARANCEProduct Information