Label: SULFAMETHOXAZOLE AND TRIMETHOPRIM injection
- NDC Code(s): 67457-778-00, 67457-778-05, 67457-779-30
- Packager: Mylan Institutional LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SULFAMETHOXAZOLE AND TRIMETHOPRIM INJECTION safely and effectively. See full prescribing information for SULFAMETHOXAZOLE AND TRIMETHOPRIM INJECTION.
SULFAMETHOXAZOLE AND TRIMETHOPRIM injection, for
intravenous use
Initial U.S. Approval: 1981RECENT MAJOR CHANGES
Warnings and Precautions, Hemophagocytic Lymphohistiocytosis (5.3) 12/2024
INDICATIONS AND USAGE
Sulfamethoxazole and trimethoprim injection is a combination of sulfamethoxazole, a sulfonamide antimicrobial, and trimethoprim, a dihydrofolate reductase inhibitor antibacterial, indicated in adults and pediatric patients two months of age and older for treatment of infections caused by designated, susceptible bacteria.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim injection and other antibacterial drugs, sulfamethoxazole and trimethoprim injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.4)
DOSAGE AND ADMINISTRATION
Dosage Guidelines For Adults and Pediatric Patients
(Two Months of Age and Older)Infection
Total Daily Dose (based on trimethoprim content)
Frequency
Duration
Pneumocystis jirovecii Pneumonia
15 to 20 mg/kg (in 3 or 4 equally divided doses)
Every 6 to 8 hours
14 days
Severe Urinary Tract Infections
8 to 10 mg/kg (in 2 to 4 equally divided doses)
Every 6, 8 or 12 hours
14 days
Shigellosis
8 to 10 mg/kg (in 2 to 4 equally divided doses)
Every 6, 8 or 12 hours
5 days
- •
- For patients with impaired renal function, a reduced dosage should be employed. (2.2)
- •
- Sulfamethoxazole and trimethoprim injection must be given by intravenous infusion over a period of 60 to 90 minutes. Rapid infusion or bolus injection must be avoided. (2.3)
- •
- Sulfamethoxazole and trimethoprim injection must be diluted in 5% dextrose in water solution prior to administration. (2.4)
- •
- Do not mix sulfamethoxazole and trimethoprim injection with other drugs or solutions. (2.4)
DOSAGE FORMS AND STRENGTHS
Injection: 80 mg/mL sulfamethoxazole and 16 mg/mL trimethoprim in 5 mL single-dose and 30 mL multiple-dose vials. (3)
CONTRAINDICATIONS
- •
- Known hypersensitivity to trimethoprim or sulfonamides (4)
- •
- History of drug-induced immune thrombocytopenia with use of trimethoprim and/or sulfonamides (4)
- •
- Documented megaloblastic anemia due to folate deficiency (4)
- •
- Pediatric patients less than two months of age (4)
- •
- Marked hepatic damage (4)
- •
- Severe renal insufficiency when renal function status cannot be monitored (4)
- •
- Concomitant administration with dofetilide (4)
WARNINGS AND PRECAUTIONS
- •
- Embryo-fetal Toxicity: Increased risk of congenital malformations. Advise patient of the potential hazards to the fetus. (5.1)
- •
- Hypersensitivity and Other Serious or Fatal Reactions: Discontinue at first appearance of skin rash or any sign of adverse reaction. (5.2)
- •
- Hemophagocytic Lymphohistiocytosis (HLH): Cases of HLH have been reported in patients treated with sulfamethoxazole-trimethoprim. If HLH is suspected, discontinue sulfamethoxazole and trimethoprim immediately and institute appropriate management. (5.3)
- •
- Thrombocytopenia: Monitor for hematologic toxicity. (5.4)
- •
- Streptococcal Infections and Rheumatic Fever: Do not use for the treatment of group A beta-hemolytic streptococcal infections. (5.5)
- •
- Clostridioides difficile-Associated Diarrhea: Evaluate if diarrhea occurs. (5.6)
- •
- Sulfite Sensitivity: May cause allergic-type reactions. (5.7)
- •
- Benzyl Alcohol Toxicity: Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates. (5.8)
- •
- Increased mortality with adjunctive leucovorin for Pneumocystis jirovecii pneumonia: Avoid concurrent use. (5.9)
- •
- Propylene glycol toxicity: Hyperosmolarity with lactic or non-gap metabolic acidosis can occur. Monitor for total intake of propylene glycol and for acid-base disturbances. (5.10)
ADVERSE REACTIONS
The most common adverse effects are gastrointestinal disturbances (nausea, vomiting, and anorexia) and allergic skin reactions (such as rash and urticaria). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- CYP2C8, CYP2C9 or OCT2 substrates: Use with caution when co-administering with sulfamethoxazole and trimethoprim. (7)
- •
- Warfarin: Monitor prothrombin time and INR. (7)
- •
- Phenytoin: Monitor serum phenytoin levels. (7)
- •
- Digoxin: Concomitant use may increase digoxin blood levels, especially in elderly patients. Monitor serum digoxin levels. (7)
- •
- Oral hypoglycemics: Concomitant use may potentiate hypoglycemic effects. Monitor blood glucose more frequently. (7)
- •
- Zidovudine: Monitor for hematologic toxicity. (7)
- •
- Procainamide: Monitor for signs of procainamide toxicity. (7)
USE IN SPECIFIC POPULATIONS
- •
- Pregnancy: Sulfamethoxazole and trimethoprim may cause fetal harm to the fetus. Use only if potential benefit justifies potential risk to the fetus. (8.1)
- •
- Lactation: Avoid breastfeeding during treatment with sulfamethoxazole and trimethoprim because of potential risk of bilirubin displacement and kernicterus. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pneumocystis jirovecii Pneumonia
1.2 Shigellosis
1.3 Urinary Tract Infections
1.4 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults and Pediatric Patients (Two Months of Age and Older)
2.2 Dosage Modifications in Patients with Impaired Renal Function
2.3 Important Administration Instructions
2.4 Method of Preparation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-fetal Toxicity
5.2 Hypersensitivity and Other Serious or Fatal Reactions
5.3 Hemophagocytic Lymphohistiocytosis
5.4 Thrombocytopenia
5.5 Streptococcal Infections and Rheumatic Fever
5.6 Clostridioides difficile-Associated Diarrhea
5.7 Sulfite Sensitivity
5.8 Benzyl Alcohol Toxicity in Pediatric Patients (“Gasping Syndrome”)
5.9 Risk Associated with Concurrent Use of Leucovorin for Pneumocystis jirovecii Pneumonia
5.10 Propylene Glycol Toxicity
5.11 Folate Deficiency
5.12 Hemolysis
5.13 Infusion Reactions
5.14 Hypoglycemia
5.15 Impaired Phenylalanine Metabolism
5.16 Porphyria and Hypothyroidism
5.17 Potential Risk in the Treatment of Pneumocystis jirovecii Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
5.18 Electrolyte Abnormalities
5.19 Monitoring of Laboratory Tests
5.20 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Interactions with Laboratory or Diagnostic Testing
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pneumocystis jirovecii Pneumonia
Sulfamethoxazole and trimethoprim injection is indicated in the treatment of Pneumocystis jirovecii pneumonia in adults and pediatric patients two months of age and older.
1.2 Shigellosis
Sulfamethoxazole and trimethoprim injection is indicated in the treatment of enteritis caused by susceptible strains of Shigella flexneri and Shigella sonnei in adults and pediatric patients two months of age and older.
1.3 Urinary Tract Infections
Sulfamethoxazole and trimethoprim injection is indicated in the treatment of severe or complicated urinary tract infections in adults and pediatric patients two months of age and older due to susceptible strains of Escherichia coli, Klebsiella species, Enterobacter species, Morganella morganii, Proteus mirabilis and Proteus vulgaris when oral administration of sulfamethoxazole and trimethoprim injection is not feasible and when the organism is not susceptible to single-agent antibacterials effective in the urinary tract.
1.4 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim injection and other antibacterial drugs, sulfamethoxazole and trimethoprim injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to empiric selection of therapy.
Although appropriate culture and susceptibility studies should be performed, therapy may be started while awaiting the results of these studies.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults and Pediatric Patients (Two Months of Age and Older)
The maximum recommended daily dose is 60 mL (960 mg trimethoprim) per day.
Table 1: Dosage in Adults and Pediatric Patients (Two Months of Age and Older) by Indication Dosage Guidelines
Infection
Total Daily Dose (based on trimethoprim content)
Frequency
Duration
Pneumocystis jirovecii Pneumonia*
15 to 20 mg/kg (in 3 or 4 equally divided doses)
Every 6 to 8 hours
14 days
Severe Urinary Tract Infections
8 to 10 mg/kg (in 2 to 4 equally divided doses)
Every 6, 8 or 12 hours
14 days
Shigellosis
8 to 10 mg/kg (in 2 to 4 equally divided doses)
Every 6, 8 or 12 hours
5 days
*A total daily dose of 10 to 15 mg/kg was sufficient in 10 adult patients with normal renal function in a published literature.1
2.2 Dosage Modifications in Patients with Impaired Renal Function
When renal function is impaired, a reduced dosage should be employed, as shown in Table 2.
Table 2: Impaired Renal Function Dosage Guidelines Creatinine Clearance (mL/min)
Recommended Dosage Regimen
Above 30
Usual standard dosage regimen
15 to 30
½ the usual dosage regimen
Below 15
Use not recommended
2.3 Important Administration Instructions
Administer the solution by intravenous infusion over a period of 60 to 90 minutes. Avoid administration by rapid infusion or bolus injection. Do NOT administer sulfamethoxazole and trimethoprim injection intramuscularly.
Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever the solution and container permit.
2.4 Method of Preparation
Dilution of Single- and Multiple-Dose Vials
Sulfamethoxazole and trimethoprim injection must be diluted. Each 5 mL should be added to 125 mL of 5% dextrose in water. After diluting with 5% dextrose in water, the solution should not be refrigerated and should be used within 6 hours.
If a dilution of 5 mL per 100 mL of 5% dextrose in water is desired, it should be used within 4 hours. In those instances where fluid restriction is desirable, each 5 mL may be added to 75 mL of 5% dextrose in water. Under these circumstances the solution should be mixed just prior to use and should be administered within 2 hours.
If upon visual inspection there is cloudiness or evidence of crystallization after mixing, the solution should be discarded and a fresh solution prepared.
Do NOT mix sulfamethoxazole and trimethoprim injection in 5% dextrose in water with drugs or solutions in the same container.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Sulfamethoxazole and trimethoprim is contraindicated in the following situations:
- •
- Known hypersensitivity to trimethoprim or sulfonamides [see Warnings and Precautions (5.2)]
- •
- History of drug-induced immune thrombocytopenia with use of trimethoprim and/or sulfonamides [see Warnings and Precautions (5.4)]
- •
- Documented megaloblastic anemia due to folate deficiency [see Warnings and Precautions (5.11)]
- •
- Pediatric patients less than two months of age [see Use in Specific Populations (8.4)]
- •
- Marked hepatic damage [see Warnings and Precautions (5.11, 5.14)]
- •
- Severe renal insufficiency when renal function status cannot be monitored [see Warnings and Precautions (5.11, 5.14)]
- •
- Concomitant administration with dofetilide2,3 [see Drug Interactions (7)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-fetal Toxicity
Some epidemiologic studies suggest that exposure to sulfamethoxazole and trimethoprim during pregnancy may be associated with an increased risk of congenital malformations, particularly neural tube defects, cardiovascular malformations, urinary tract defects, oral clefts, and club foot. If sulfamethoxazole and trimethoprim is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be advised of the potential hazards to the fetus [see Use in Specific Populations (8.1)].
5.2 Hypersensitivity and Other Serious or Fatal Reactions
Fatalities and serious adverse reactions including severe cutaneous adverse reactions (SCARs), including Stevens-Johnson Syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), acute febrile neutrophilic dermatosis (AFND), acute generalized erythematous pustulosis (AGEP); fulminant hepatic necrosis; agranulocytosis, aplastic anemia and other blood dyscrasias; acute and delayed lung injury; anaphylaxis and circulatory shock have occurred with the administration of sulfamethoxazole and trimethoprim products, including sulfamethoxazole and trimethoprim [see Adverse Reactions (6.1)].
Cough, shortness of breath and pulmonary infiltrates potentially representing hypersensitivity reactions of the respiratory tract have been reported in association with sulfamethoxazole and trimethoprim treatment.
Other severe pulmonary adverse reactions occurring within days to week of sulfamethoxazole and trimethoprim initiation and resulting in prolonged respiratory failure requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO), lung transplantation or death have also been reported in patients and otherwise healthy individuals treated with sulfamethoxazole and trimethoprim products.
Circulatory shock with fever, severe hypotension, and confusion requiring intravenous fluid resuscitation and vasopressors has occurred within minutes to hours of re-challenge with sulfamethoxazole and trimethoprim products, including sulfamethoxazole and trimethoprim, in patients with history of recent (days to weeks) exposure to sulfamethoxazole and trimethoprim.
Sulfamethoxazole and trimethoprim should be discontinued at the first appearance of skin rash or any sign of a serious adverse reaction. A skin rash may be followed by more severe reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, DRESS, AFND, AGEP, hepatic necrosis or serious blood disorder. Clinical signs, such as rash, pharyngitis, fever, arthralgia, cough, chest pain, dyspnea, pallor, purpura or jaundice may be early indications of serious reactions.
5.3 Hemophagocytic Lymphohistiocytosis
Cases of hemophagocytic lymphohistiocytosis (HLH) have been reported in patients treated with sulfamethoxazole-trimethoprim. HLH is a life-threatening syndrome of pathologic immune activation characterized by clinical signs and symptoms of extreme systemic inflammation. Signs and symptoms of HLH may include fever, hepatosplenomegaly, rash, lymphadenopathy, neurologic symptoms, cytopenias, high serum ferritin, hypertriglyceridemia, and liver enzyme and coagulation abnormalities. If HLH is suspected, discontinue sulfamethoxazole and trimethoprim immediately and institute appropriate management.
5.4 Thrombocytopenia
Sulfamethoxazole and trimethoprim-induced thrombocytopenia may be an immune-mediated disorder. Severe cases of thrombocytopenia that are fatal or life threatening have been reported. Monitor patients for hematologic toxicity. Thrombocytopenia usually resolves within a week upon discontinuation of sulfamethoxazole and trimethoprim.
5.5 Streptococcal Infections and Rheumatic Fever
Avoid use of sulfamethoxazole and trimethoprim in the treatment of streptococcal pharyngitis. Clinical studies have documented that patients with group A β-hemolytic streptococcal tonsillopharyngitis have a greater incidence of bacteriologic failure when treated with sulfamethoxazole and trimethoprim than do those patients treated with penicillin, as evidenced by failure to eradicate this organism from the tonsillopharyngeal area. Therefore, sulfamethoxazole and trimethoprim will not prevent sequelae such as rheumatic fever.
5.6 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including sulfamethoxazole and trimethoprim, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.7 Sulfite Sensitivity
Sulfamethoxazole and trimethoprim contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
5.8 Benzyl Alcohol Toxicity in Pediatric Patients (“Gasping Syndrome”)
Sulfamethoxazole and trimethoprim contains benzyl alcohol as a preservative. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low birth weight infants treated with benzyl alcohol-preserved formulations in infusion solutions, including sulfamethoxazole and trimethoprim. The “gasping syndrome” is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. Sulfamethoxazole and trimethoprim is contraindicated in pediatric patients less than two months of age [see Contraindications (4)].
When prescribing sulfamethoxazole and trimethoprim in pediatric patients (two months of age and older), consider the combined daily metabolic load of benzyl alcohol from all sources including sulfamethoxazole and trimethoprim (contains 10 mg of benzyl alcohol per mL) and other drugs containing benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known [see Use in Specific Populations (8.4)].
5.9 Risk Associated with Concurrent Use of Leucovorin for Pneumocystis jirovecii Pneumonia
Treatment failure and excess mortality were observed when sulfamethoxazole and trimethoprim was used concomitantly with leucovorin for the treatment of HIV positive patients with P. jirovecii pneumonia in a randomized placebo-controlled trial.4 Avoid coadministration of sulfamethoxazole and trimethoprim and leucovorin during treatment of P. jirovecii pneumonia.
5.10 Propylene Glycol Toxicity
Sulfamethoxazole and trimethoprim contains propylene glycol as a solvent (40% v/v). When administered at high doses as for the treatment of P. jirovecii pneumonia and concomitantly with other products that contain propylene glycol, hyperosmolarity with anion gap metabolic acidosis, including lactic acidosis can occur. Propylene glycol toxicity can lead to acute kidney injury, CNS toxicity, and multi-organ failure. Monitor for the total daily intake of propylene glycol from all sources and for acid-base disturbances. Discontinue sulfamethoxazole and trimethoprim if propylene glycol toxicity is suspected [see Adverse Reactions (6)].
5.11 Folate Deficiency
Avoid use of sulfamethoxazole and trimethoprim in patients with impaired renal or hepatic function, in those with possible folate deficiency (e.g., the elderly, chronic alcoholics, patients receiving anticonvulsant therapy, patients with malabsorption syndrome, and patients in malnutrition states) and in those with severe allergies or bronchial asthma.
Hematologic changes indicative of folic acid deficiency may occur in elderly patients or in patients with pre-existing folic acid deficiency or kidney failure. These effects are reversible by folinic acid therapy [see Use in Specific Populations (8.5)].
5.12 Hemolysis
In glucose-6-phosphate dehydrogenase deficient individuals, hemolysis may occur. This reaction is frequently dose-related.
5.13 Infusion Reactions
Local irritation and inflammation due to extravascular infiltration of the infusion have been observed with sulfamethoxazole and trimethoprim. If these occur the infusion should be discontinued and restarted at another site.
5.14 Hypoglycemia
Cases of hypoglycemia in non-diabetic patients treated with sulfamethoxazole and trimethoprim have been seen, usually occurring after a few days of therapy. Patients with renal dysfunction, liver disease, malnutrition or those receiving high doses of sulfamethoxazole and trimethoprim are particularly at risk.
5.15 Impaired Phenylalanine Metabolism
The trimethoprim component of sulfamethoxazole and trimethoprim has been noted to impair phenylalanine metabolism, but this is of no significance in phenylketonuric patients on appropriate dietary restriction.
5.16 Porphyria and Hypothyroidism
Like other drugs containing sulfonamides, sulfamethoxazole and trimethoprim can precipitate porphyria crisis and hypothyroidism. Avoid use of sulfamethoxazole and trimethoprim in patients with porphyria or thyroid dysfunction.
5.17 Potential Risk in the Treatment of Pneumocystis jirovecii Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
AIDS patients may not tolerate or respond to sulfamethoxazole and trimethoprim in the same manner as non-AIDS patients. The incidence of adverse reactions, particularly rash, fever, leukopenia, and elevated aminotransferase (transaminase) values, with sulfamethoxazole and trimethoprim therapy in AIDS patients who are being treated for P. jirovecii pneumonia has been reported to be increased compared with the incidence normally associated with the use of sulfamethoxazole and trimethoprim in non-AIDS patients. If a patient develops skin rash, fever, leukopenia or any sign of an adverse reaction, re-evaluate benefit-risk of continuing therapy or re-challenge with sulfamethoxazole and trimethoprim [see Warnings and Precautions (5.2)].
Avoid coadministration of sulfamethoxazole and trimethoprim and leucovorin during treatment of P. jirovecii pneumonia [see Warnings and Precautions (5.9)].
5.18 Electrolyte Abnormalities
Hyperkalemia
High dosage of trimethoprim, as used in patients with P. jirovecii pneumonia, induces a progressive but reversible increase of serum potassium concentrations in a substantial number of patients. Even treatment with recommended doses may cause hyperkalemia when trimethoprim is administered to patients with underlying disorders of potassium metabolism, with renal insufficiency, or if drugs known to induce hyperkalemia are given concomitantly. Close monitoring of serum potassium is warranted in these patients.
Hyponatremia
Severe and symptomatic hyponatremia can occur in patients receiving sulfamethoxazole and trimethoprim, particularly for the treatment of P. jirovecii pneumonia. Evaluation for hyponatremia and appropriate correction is necessary in symptomatic patients to prevent life-threatening complications.
5.19 Monitoring of Laboratory Tests
Complete blood counts and clinical chemistry testing should be done frequently in patients receiving sulfamethoxazole and trimethoprim. Discontinue sulfamethoxazole and trimethoprim if a significant electrolyte abnormality, renal insufficiency or reduction in the count of any formed blood element is noted. Perform urinalyses with careful microscopic examination and renal function tests during therapy, particularly for those patients with impaired renal function.
5.20 Development of Drug-Resistant Bacteria
Prescribing sulfamethoxazole and trimethoprim in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- •
- Embryo-fetal Toxicity [see Warnings and Precautions (5.1)]
- •
- Hypersensitivity and Other Fatal Reactions [see Warnings and Precautions (5.2)]
- •
- Thrombocytopenia [see Warnings and Precautions (5.4)]
- •
- Clostridioides difficile-Associated Diarrhea [see Warnings and Precautions (5.6)]
- •
- Sulfite Sensitivity [see Warnings and Precautions (5.7)]
- •
- Risk Associated with Concurrent Use of Leucovorin for Pneumocystis jirovecii Pneumonia [see Warnings and Precautions (5.9)]
- •
- Propylene Glycol Toxicity [see Warnings and Precautions (5.10)]
- •
- Infusion Reactions [see Warnings and Precautions (5.13)]
- •
- Hypoglycemia [see Warnings and Precautions (5.14)]
- •
- Electrolyte Abnormalities [see Warnings and Precautions (5.18)]
6.1 Clinical Trials Experience
The following adverse reactions associated with the use of sulfamethoxazole and trimethoprim injection or sulfamethoxazole and trimethoprim were identified in clinical trials, postmarketing or published reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most common adverse reactions are gastrointestinal disturbances (nausea, vomiting, and anorexia) and allergic skin reactions (such as rash and urticaria). Fatalities and serious adverse reactions, including severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), acute febrile neutrophilic dermatosis (AFND), acute generalized erythematous pustulosis (AGEP); fulminant hepatic necrosis; agranulocytosis, aplastic anemia and other blood dyscrasias; acute and delayed lung injury; anaphylaxis and circulatory shock have occurred with the administration of sulfamethoxazole and trimethoprim products, including sulfamethoxazole and trimethoprim [see Warnings and Precautions (5.2)].
Local reaction, pain and slight irritation on intravenous (IV) administration are infrequent. Thrombophlebitis has been observed.
Table 3: Adverse Reactions Reported with Sulfamethoxazole and Trimethoprim Injection Body System
Adverse Reactions
Hematologic
Agranulocytosis, aplastic anemia, thrombocytopenia, leukopenia, neutropenia, hemolytic anemia, megaloblastic anemia, hypoprothrombinemia, methemoglobinemia, eosinophilia, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura.
Allergic/Immune Reactions
Stevens-Johnson syndrome, toxic epidermal necrolysis, anaphylaxis, allergic myocarditis, erythema multiforme, exfoliative dermatitis, angioedema, drug fever, chills, Henoch-Schoenlein purpura, serum sickness-like syndrome, generalized allergic reactions, generalized skin eruptions, photosensitivity, conjunctival and scleral injection, pruritus, urticaria, rash, periarteritis nodosa, hemophagocytic lymphohistiocytosis (HLH), systemic lupus erythematosus, drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized erythematous pustulosis (AGEP), and acute febrile neutrophilic dermatosis (AFND) [see Warnings and Precautions (5.2 and 5.3)].
Gastrointestinal
Hepatitis (including cholestatic jaundice and hepatic necrosis), elevation of serum transaminase and bilirubin, pseudomembranous enterocolitis, pancreatitis, stomatitis, glossitis, nausea, emesis, abdominal pain, diarrhea, anorexia.
Genitourinary
Renal failure, interstitial nephritis, BUN and serum creatinine elevation, renal insufficiency, oliguria and anuria, crystalluria and nephrotoxicity in association with cyclosporine.
Metabolic and Nutritional
Hyperkalemia, hyponatremia [see Warnings and Precautions (5.18)], metabolic acidosis.
Neurologic
Aseptic meningitis, convulsions, peripheral neuritis, ataxia, vertigo, tinnitus, headache.
Psychiatric
Hallucinations, depression, apathy, nervousness.
Endocrine
The sulfonamides bear certain chemical similarities to some goitrogens, diuretics (acetazolamide and the thiazides) and oral hypoglycemic agents. Cross-sensitivity may exist with these agents. Diuresis and hypoglycemia have occurred.
Musculoskeletal
Arthralgia, Myalgia, rhabdomyolysis
Respiratory
Cough, shortness of breath and pulmonary infiltrates, acute eosinophilic pneumonia, acute and delayed lung injury, interstitial lung disease, acute respiratory failure [see Warnings and Precautions (5.2)].
Cardiovascular System
QT prolongation resulting in ventricular tachycardia and torsades de pointes, circulatory shock [see Warnings and Precautions (5.2)].
Miscellaneous
Weakness, fatigue, insomnia.
Eye Disorders
Uveitis5
-
7 DRUG INTERACTIONS
Potential for Sulfamethoxazole and Trimethoprim to Affect Other Drugs
Trimethoprim is an inhibitor of CYP2C8 as well as OCT2 transporter. Sulfamethoxazole is an inhibitor of CYP2C9. Avoid coadministration of sulfamethoxazole and trimethoprim with drugs that are substrates of CYP2C8 and 2C9 or OCT2.
Table 4: Drug Interactions with Sulfamethoxazole and Trimethoprim Injection Drug(s)
Recommendation
Comments
Diuretics
Avoid concurrent use
In elderly patients concurrently receiving certain diuretics, primarily thiazides, an increased incidence of thrombocytopenia with purpura has been reported.
Warfarin
Monitor prothrombin time and INR
It has been reported that sulfamethoxazole and trimethoprim may prolong the prothrombin time in patients who are receiving the anticoagulant warfarin (a CYP2C9 substrate). This interaction should be kept in mind when sulfamethoxazole and trimethoprim is given to patients already on anticoagulant therapy, and the coagulation time should be reassessed.
Phenytoin
Monitor serum phenytoin levels
Sulfamethoxazole and trimethoprim may inhibit the hepatic metabolism of phenytoin (a CYP2C9 substrate). Sulfamethoxazole and trimethoprim, given at a common clinical dosage, increased the phenytoin half-life by 39% and decreased the phenytoin metabolic clearance rate by 27%. When administering these drugs concurrently, one should be alert for possible excessive phenytoin effect.
Methotrexate
Avoid concurrent use
Sulfonamides can also displace methotrexate from plasma protein binding sites and can compete with the renal transport of methotrexate, thus increasing free methotrexate concentrations.
Cyclosporine
Avoid concurrent use
There have been reports of marked but reversible nephrotoxicity with coadministration of sulfamethoxazole and trimethoprim and cyclosporine in renal transplant recipients.
Digoxin
Monitor serum digoxin levels
Increased digoxin blood levels can occur with concomitant sulfamethoxazole and trimethoprim therapy, especially in elderly patients
Indomethacin
Avoid concurrent use
Increased sulfamethoxazole blood levels may occur in patients who are also receiving indomethacin.
Pyrimethamine
Avoid concurrent use
Occasional reports suggest that patients receiving pyrimethamine as malaria prophylaxis in doses exceeding 25 mg weekly may develop megaloblastic anemia if sulfamethoxazole and trimethoprim is prescribed.
Tricyclic Antidepressants (TCAs)
Monitor therapeutic response and adjust dose of TCA accordingly
The efficacy of tricyclic antidepressants can decrease when coadministered with sulfamethoxazole and trimethoprim.
Oral hypoglycemics
Monitor blood
glucose more frequently
Like other sulfonamide-containing drugs, sulfamethoxazole and trimethoprim potentiates the effect of oral hypoglycemic that are metabolized by CYP2C8 (e.g., pioglitazone, repaglinide, and rosiglitazone) or CYP2C9 (e.g., glipizide and glyburide) or eliminated renally via OCT2 (e.g., metformin). Additional monitoring of blood glucose may be warranted.
Amantadine
Avoid concurrent use
In the literature, a single case of toxic delirium has been reported after concomitant intake of sulfamethoxazole and trimethoprim and amantadine (an OCT2 substrate). Cases of interactions with other OCT2 substrates, memantine and metformin, have also been reported.
Angiotensin Converting Enzyme Inhibitors
Avoid concurrent use
In the literature, three cases of hyperkalemia in elderly patients have been reported after concomitant intake of sulfamethoxazole and trimethoprim and an angiotensin converting enzyme inhibitor.6,7
Zidovudine
Monitor for hematologic toxicity
Zidovudine and sulfamethoxazole and trimethoprim are known to induce hematological abnormalities. Hence, there is potential for an additive myelotoxicity when coadministered.8
Dofetilide
Concurrent administration is contraindicated
Elevated plasma concentrations of dofetilide have been reported following concurrent administration of trimethoprim and dofetilide. Increased plasma concentrations of dofetilide may cause serious ventricular arrhythmias associated with QT interval prolongation, including torsade de pointes.2,3
Procainamide
Closely monitor for clinical and ECG signs of procainamide toxicity and/or procainamide plasma concentration if available
Trimethoprim increases the plasma concentrations of procainamide and its active N-acetyl metabolite (NAPA) when trimethoprim and procainamide are coadministered. The increased procainamide and NAPA plasma concentrations that resulted from the pharmacokinetic interaction with trimethoprim are associated with further prolongation of the QTc interval.9
7.1 Interactions with Laboratory or Diagnostic Testing
Sulfamethoxazole and trimethoprim, specifically the trimethoprim component, can interfere with a serum methotrexate assay as determined by the competitive binding protein technique (CBPA) when a bacterial dihydrofolate reductase is used as the binding protein. No interference occurs, however, if methotrexate is measured by a radioimmunoassay (RIA).
The presence of sulfamethoxazole and trimethoprim may also interfere with the Jaffé alkaline picrate reaction assay for creatinine, resulting in overestimations of about 10% in the range of normal values.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Sulfamethoxazole and trimethoprim may cause fetal harm if administered to a pregnant woman. Some epidemiologic studies suggest that exposure to sulfamethoxazole and trimethoprim during pregnancy may be associated with an increased risk of congenital malformations, particularly neural tube defects, cardiovascular abnormalities, urinary tract defects, oral clefts, and club foot (see Human Data).
One of 3 rat studies showed cleft palate at doses approximately 5 times the recommended human dose on a body surface area basis; the other 2 studies did not show teratogenicity at similar doses. Studies in pregnant rabbits showed increased fetal loss at approximately 6 times the human dose on a body surface area basis (see Animal Data).
The estimated background risk of major birth defects and miscarriages for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Advise pregnant women of the potential harm of sulfamethoxazole and trimethoprim to the fetus (see Clinical Considerations).
Clinical Considerations
Disease-associated Maternal and/or Embryo/Fetal Risk
Urinary tract infection in pregnancy is associated with adverse perinatal outcomes such as preterm birth, low birth weight, and pre-eclampsia, and increased mortality to the pregnant woman. P. jirovecii pneumonia in pregnancy is associated with preterm birth and increased morbidity and mortality for the pregnant woman. Sulfamethoxazole and trimethoprim should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Data
Human Data
While there are no large, prospective, well-controlled studies in pregnant women and their babies, some retrospective epidemiologic studies suggest an association between first trimester exposure to sulfamethoxazole and trimethoprim with an increased risk of congenital malformations, particularly neural tube defects, cardiovascular abnormalities, urinary tract defects, oral clefts, and club foot. These studies, however, were limited by the small number of exposed cases and the lack of adjustment for multiple statistical comparisons and confounders. These studies are further limited by recall, selection, and information biases, and by limited generalizability of their findings. Lastly, outcome measures varied between studies, limiting cross-study comparisons.
Alternatively, other epidemiologic studies did not detect statistically significant associations between sulfamethoxazole and trimethoprim exposure and specific malformations. Brumfitt and Pursell,10 in a retrospective study, reported the outcome of 186 pregnancies during which the mother received either placebo or oral trimethoprim and sulfamethoxazole. The incidence of congenital abnormalities was 4.5% (3 of 66) in those who received placebo and 3.3% (4 of 120) in those receiving trimethoprim and sulfamethoxazole. There were no abnormalities in the 10 children whose mothers received the drug during the first trimester. In a separate survey, Brumfitt and Pursell also found no congenital abnormalities in 35 children whose mothers had received oral trimethoprim and sulfamethoxazole at the time of conception or shortly thereafter.
Animal Data
In rats, oral doses of either 533 mg/kg sulfamethoxazole or 200 mg/kg trimethoprim produced teratologic effects manifested mainly as cleft palates. These doses are approximately 5 and 6 times the recommended human total daily dose on a body surface area basis. In two studies in rats, no teratology was observed when 512 mg/kg of sulfamethoxazole was used in combination with 128 mg/kg of trimethoprim. In some rabbit studies, an overall increase in fetal loss (dead and resorbed conceptuses) was associated with doses of trimethoprim 6 times the human therapeutic dose based on body surface area.
8.2 Lactation
Risk Summary
Levels of sulfamethoxazole and trimethoprim in breast milk are approximately 2 to 5% of the recommended daily dose for pediatric patients over two months of age. There is no information regarding the effect of sulfamethoxazole and trimethoprim on the breastfed infant or the effect on milk production. Because of the potential risk of bilirubin displacement and kernicterus on the breastfed child [see Contraindications (4)], advise women to avoid breastfeeding during treatment with sulfamethoxazole and trimethoprim.
8.4 Pediatric Use
Sulfamethoxazole and trimethoprim is contraindicated in pediatric patients younger than two months of age because of the potential risk of bilirubin displacement and kernicterus [see Contraindications (4)].
Serious adverse reactions including fatal reactions and the “gasping syndrome” occurred in premature neonates and low birth weight infants in the neonatal intensive care unit who received benzyl alcohol as a preservative in infusion solutions. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day produced high levels of benzyl alcohol and its metabolites in the blood and urine (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low-birth weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol.
When prescribing sulfamethoxazole and trimethoprim in pediatric patients consider the combined daily metabolic load of benzyl alcohol from all sources including sulfamethoxazole and trimethoprim (sulfamethoxazole and trimethoprim injection contains 10 mg of benzyl alcohol per mL) and other drugs containing benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known [see Warnings and Precautions (5.8)].
8.5 Geriatric Use
Clinical studies of sulfamethoxazole and trimethoprim did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
There may be an increased risk of severe adverse reactions in elderly patients, particularly when complicating conditions exist, e.g., impaired kidney and/or liver function, or concomitant use of other drugs. Severe skin reactions, generalized bone marrow suppression [see Warnings and Precautions (5.11), Adverse Reactions (6.1)], a specific decrease in platelets (with or without purpura), and hyperkalemia are the most frequently reported severe adverse reactions in elderly patients.
In those concurrently receiving certain diuretics, primarily thiazides, an increased incidence of thrombocytopenia with purpura has been reported. Increased digoxin blood levels can occur with concomitant sulfamethoxazole and trimethoprim therapy, especially in elderly patients. Serum digoxin levels should be monitored [see Drug Interactions (7)].
Hematologic changes indicative of folic acid deficiency may occur in elderly patients. These effects are reversible by folinic acid therapy. Appropriate dosage adjustments should be made for patients with impaired kidney function and duration of use should be as short as possible to minimize risks of undesired reactions [see Dosage and Administration (2.2)].
The trimethoprim component of sulfamethoxazole and trimethoprim may cause hyperkalemia when administered to patients with underlying disorders of potassium metabolism, with renal insufficiency or when given concomitantly with drugs known to induce hyperkalemia, such as angiotensin converting enzyme inhibitors. Close monitoring of serum potassium is warranted in these patients. Discontinuation of sulfamethoxazole and trimethoprim treatment is recommended to help lower potassium serum levels.
Pharmacokinetics parameters for sulfamethoxazole were similar for geriatric subjects and younger adult subjects. The mean maximum serum trimethoprim concentration was higher and mean renal clearance of trimethoprim was lower in geriatric subjects compared with younger subjects [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Acute
Since there has been no extensive experience in humans with single doses of sulfamethoxazole and trimethoprim in excess of 25 mL (400 mg trimethoprim and 2000 mg sulfamethoxazole), the maximum tolerated dose in humans is unknown.
Signs and symptoms of overdosage reported with sulfonamides include anorexia, colic, nausea, vomiting, dizziness, headache, drowsiness and unconsciousness. Pyrexia, hematuria and crystalluria may be noted. Blood dyscrasias and jaundice are potential late manifestations of overdosage.
Signs of acute overdosage with trimethoprim include nausea, vomiting, dizziness, headache, mental depression, confusion and bone marrow depression.
General principles of treatment include the administration of intravenous fluids if urine output is low and renal function is normal. Acidification of the urine will increase renal elimination of trimethoprim. The patient should be monitored with blood counts and appropriate blood chemistries, including electrolytes. If a significant blood dyscrasia or jaundice occurs, specific therapy should be instituted for these complications. Peritoneal dialysis is not effective and hemodialysis is only moderately effective in eliminating trimethoprim and sulfamethoxazole.
Chronic
Use of sulfamethoxazole and trimethoprim at high doses and/or for extended periods of time may cause bone marrow depression manifested as thrombocytopenia, leukopenia and/or megaloblastic anemia. If signs of bone marrow depression occur, the patient should be given leucovorin 5 to 15 mg daily until normal hematopoiesis is restored.
-
11 DESCRIPTION
Sulfamethoxazole and trimethoprim injection USP, a sterile, clear, colorless or slightly yellow solution for intravenous infusion only, is a combination of sulfamethoxazole, a sulfonamide antimicrobial, and trimethoprim, a dihydrofolate reductase inhibitor antibacterial. Each mL contains 16 mg trimethoprim, USP and 80 mg sulfamethoxazole, USP compounded with propylene glycol 400 mg (38.6% v/v and 40.0% w/v); dehydrated alcohol 100 mg (12.3% v/v and 10.0% w/v); diethanolamine 3 mg (0.3% v/v and 0.3% w/v); benzyl alcohol 10 mg (1.0% v/v and 1.0% w/v) as a preservative; sodium metabisulfite 1 mg (0.1% w/v) as an antioxidant; water for injection q.s.; air replaced with nitrogen; pH adjusted with sodium hydroxide and/or hydrochloric acid if necessary. pH 9.5 to 10.5.
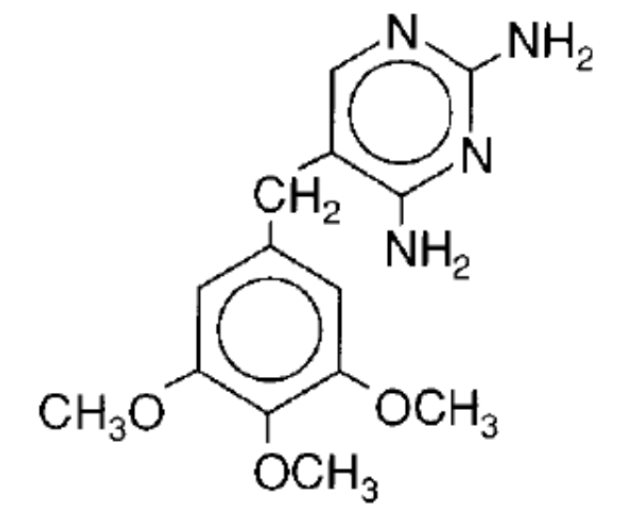
Trimethoprim, USP is 2,4-diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine. It is a white to light yellow, odorless, bitter compound with a molecular weight of 290.3 and the following structural formula:
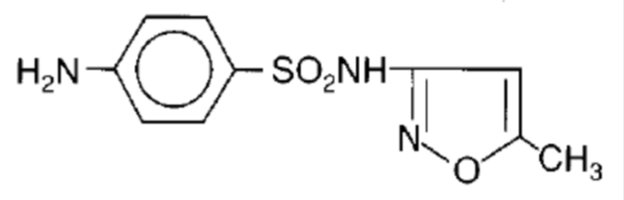
Sulfamethoxazole, USP is N1-(5-methyl-3-isoxazolyl) sulfanilamide. It is an almost white, odorless, tasteless compound with a molecular weight of 253.28 and the following structural formula:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sulfamethoxazole and trimethoprim is an antimicrobial drug [see Microbiology (12.4)].
12.3 Pharmacokinetics
Following a 1-hour intravenous infusion of a single dose of 160 mg trimethoprim and 800 mg sulfamethoxazole to 11 patients whose weight ranged from 105 lbs to 165 lbs (mean, 143 lbs), the peak plasma concentrations of trimethoprim and sulfamethoxazole were 3.4 ± 0.3 mcg/mL and 46.3 ± 2.7 mcg/mL, respectively. Following repeated intravenous administration of the same dose at 8-hour intervals, the mean plasma concentrations just prior to and immediately after each infusion at steady-state were 5.6 ± 0.6 mcg/mL and 8.8 ± 0.9 mcg/mL for trimethoprim and 70.6 ± 7.3 mcg/mL and 105.6 ± 10.9 mcg/mL for sulfamethoxazole. The mean plasma half-life was 11.3 ± 0.7 hours for trimethoprim and 12.8 ± 1.8 hours for sulfamethoxazole. All of these 11 patients had normal renal function, and their ages ranged from 17 to 78 years (median, 60 years).11
Pharmacokinetic studies in children and adults suggest an age-dependent half-life of trimethoprim, as indicated in Table 5.12
Table 5: Half-life of Trimethoprim (TMP) in Pediatric Patients and Adults Age (years)
No. of Patients
Mean TMP Half-life (hours)
<1
2
7.67
1 to 10
9
5.49
10 to 20
5
8.19
20 to 63
6
12.82
Patients with severely impaired renal function exhibit an increase in the half-lives of both components, requiring dosage regimen adjustment [see Dosage and Administration (2.2)].
Distribution
Both trimethoprim and sulfamethoxazole exist in the blood as unbound, protein-bound and metabolized forms; sulfamethoxazole also exists as the conjugated form.
Approximately 44% of trimethoprim and 70% of sulfamethoxazole are bound to plasma proteins. The presence of 10 mg percent sulfamethoxazole in plasma decreases the protein binding of trimethoprim by an insignificant degree; trimethoprim does not influence the protein binding of sulfamethoxazole.
Both trimethoprim and sulfamethoxazole distribute to sputum and vaginal fluid; trimethoprim also distributes to bronchial secretions, and both pass the placental barrier and are excreted in breast milk.
Elimination
Metabolism
Sulfamethoxazole is metabolized in humans to at least 5 metabolites: the N4-acetyl-, N4-hydroxy-, 5-methylhydroxy-, N4-acetyl-5-methylhydroxy-sulfamethoxazole metabolites, and an N-glucuronide conjugate. The formation of N4-hydroxy metabolite is mediated via CYP2C9.
Trimethoprim is metabolized in vitro to 11 different metabolites, of which, five are glutathione adducts and six are oxidative metabolites, including the major metabolites, 1- and 3-oxides and the 3- and 4-hydroxy derivatives.
The free forms of trimethoprim and sulfamethoxazole are considered to be the therapeutically active forms. In vitro studies suggest that trimethoprim is a substrate of P-glycoprotein, OCT1 and OCT2, and that sulfamethoxazole is not a substrate of P-glycoprotein.
Excretion
Excretion of trimethoprim and sulfamethoxazole is primarily by the kidneys through both glomerular filtration and tubular secretion. Urine concentrations of both trimethoprim and sulfamethoxazole are considerably higher than are the concentrations in the blood. The percent of dose excreted in urine over a 12-hour period following the intravenous administration of the first dose of 240 mg of trimethoprim and 1200 mg of sulfamethoxazole on day 1 ranged from 17% to 42.4% as free trimethoprim; 7% to 12.7% as free sulfamethoxazole; and 36.7% to 56% as total (free plus the N4-acetylated metabolite) sulfamethoxazole. When administered together as sulfamethoxazole and trimethoprim, neither trimethoprim nor sulfamethoxazole affects the urinary excretion pattern of the other.
Specific Populations
Geriatric Patients: The pharmacokinetics of sulfamethoxazole 800 mg and trimethoprim 160 mg were studied in six geriatric subjects (mean age: 78.6 years) and six young healthy subjects (mean age: 29.3 years) using a non-US approved formulation. Pharmacokinetic values for sulfamethoxazole in geriatric subjects were similar to those observed in young adult subjects. The mean renal clearance of trimethoprim was significantly lower in geriatric subjects compared with young adult subjects (19 mL/h/kg vs. 55 mL/h/kg). However, after normalizing by body weight, the apparent total body clearance of trimethoprim was on average 19% lower in geriatric subjects compared with young adult subjects.
12.4 Microbiology
Mechanism of Action
Sulfamethoxazole inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid (PABA). Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the required enzyme, dihydrofolate reductase. Thus, sulfamethoxazole and trimethoprim blocks two consecutive steps in the biosynthesis of nucleic acids and proteins essential to many bacteria.
Resistance
In vitro studies have shown that bacterial resistance develops more slowly with both sulfamethoxazole and trimethoprim in combination than with either sulfamethoxazole or trimethoprim alone.
Antimicrobial Activity
Sulfamethoxazole and trimethoprim has been shown to be active against most isolates of the following microorganisms, both in in vitro and in clinical infections [see Indications and Usage (1)].
Aerobic gram-negative bacteria
Escherichia coli
Klebsiella species
Enterobacter species
Morganella morganii
Proteus mirabilis
Proteus vulgaris
Shigella flexneri
Shigella sonnei
Other Microorganisms
Pneumocystis jirovecii
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for sulfamethoxazole and trimethoprim against isolates of similar genus or organism group. However, the efficacy of sulfamethoxazole and trimethoprim in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Sulfamethoxazole was not carcinogenic when assessed in a 26-week tumorigenic mouse (Tg-rasH2) study at doses up to 400 mg/kg/day sulfamethoxazole; equivalent to 2-fold the human systemic exposure (at a daily dose of 800 mg sulfamethoxazole twice a day ).
Mutagenesis
In vitro reverse mutation bacterial tests according to the standard protocol have not been performed with sulfamethoxazole and trimethoprim in combination. An in vitro chromosomal aberration test in human lymphocytes with sulfamethoxazole/trimethoprim was negative. In in vitro and in vivo tests in animal species, sulfamethoxazole/trimethoprim did not damage chromosomes. In vivo micronucleus assays were positive following oral administration of sulfamethoxazole/trimethoprim. Observations of leukocytes obtained from patients treated with sulfamethoxazole and trimethoprim revealed no chromosomal abnormalities.
Sulfamethoxazole alone was positive in an in vitro reverse mutation bacterial assay and in in vitro micronucleus assays using cultured human lymphocytes.
Trimethoprim alone was negative in in vitro reverse mutation bacterial assays and in in vitro chromosomal aberration assays with Chinese Hamster ovary or lung cells with or without S9 activation. In in vitro Comet, micronucleus and chromosomal damage assays using cultured human lymphocytes, trimethoprim was positive. In mice following oral administration of trimethoprim, no DNA damage in Comet assays of liver, kidney, lung, spleen, or bone marrow was recorded.
-
15 REFERENCES
- 1.
- Winston DJ, Lau WK, Gale RP, Young LS. Trimethoprim-Sulfamethoxazole for the Treatment of Pneumocystis carinii pneumonia. Ann Intern Med. June 1980;92:762-769.
- 2.
- Al-Khatib SM, LaPointe N, Kramer JM, Califf RM. What Clinicians Should Know About the QT Interval. JAMA. 2003;289(16):2120-2127.
- 3.
- Boyer EW, Stork C, Wang RY. Review: The Pharmacology and Toxicology of Dofetilide. Int J Med Toxicol. 2001;4(2):16.
- 4.
- Safrin S, Lee BL, Sande MA. Adjunctive folinic acid with trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia in AIDS patients is associated with an increased risk of therapeutic failure and death. J Infect Dis. Oct 1994;170(4):912-7.
- 5.
- London NJ, Garg SJ, Moorthy RS, Cunningham ET. Drug-induced uveitis. J Ophthalmic Inflamm Infect. 2013;3:43.
- 6.
- Marinella MA. Trimethoprim-induced hyperkalemia: An analysis of reported cases. Gerontol. 1999;45:209–212.
- 7.
- Margassery S, Bastani B. Life threatening hyperkalemia and acidosis secondary to trimethoprim-sulfamethoxazole treatment. J. Nephrol. 2001;14(5):410-414.
- 8.
- Moh R, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Côte d’Ivoire. Antivir Ther. 2005;10(5):615-24.
- 9.
- Kosoglou T, Rocci ML Jr, Vlasses PH. Trimethoprim alters the disposition of procainamide and N-acetylprocainamide. Clin Pharmacol Ther. Oct 1988;44(4):467-77.
- 10.
- Brumfitt W, Pursell R. Trimethoprim/Sulfamethoxazole in the Treatment of Bacteriuria in Women. J Infect Dis. Nov 1973;128 (Suppl): S657-S663.
- 11.
- Grose WE, Bodey GP, Loo TL. Clinical Pharmacology of Intravenously Administered Trimethoprim-Sulfamethoxazole. Antimicrob Agents Chemother. Mar 1979;15:447-451.
- 12.
- Siber GR, Gorham C, Durbin W, Lesko L, Levin MJ. Pharmacology of Intravenous Trimethoprim-Sulfamethoxazole in Children and Adults. Current Chemotherapy and Infectious Diseases. American Society for Microbiology, Washington, D.C. 1980; Vol. 1, pp. 691-692.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Sulfamethoxazole and trimethoprim injection, USP is supplied as follows:
5 mL Single-Dose Vials, containing 80 mg trimethoprim (16 mg/mL) and 400 mg sulfamethoxazole (80 mg/mL) for infusion with 5% dextrose in water.
- •
- 5 mL Vial: NDC 67457-778-00
- •
- 5 mL Vial (Box of 10): NDC 67457-778-05
30 mL Multiple-Dose Vials, each 5 mL containing 80 mg trimethoprim (16 mg/mL) and 400 mg sulfamethoxazole (80 mg/mL) for infusion with 5% dextrose in water.
- •
- 30 mL Vial (Box of 1): NDC 67457-779-30
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. DO NOT REFRIGERATE.
-
17 PATIENT COUNSELING INFORMATION
Embryo–fetal Toxicity
Advise female patients of reproductive potential that sulfamethoxazole and trimethoprim can cause fetal harm and to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Hypersensitivity and Other Serious or Fatal Reactions
Advise patients to stop taking sulfamethoxazole and trimethoprim immediately if they experience any clinical signs such as rash, pharyngitis, fever, arthralgia, cough, chest pain, dyspnea, pallor, purpura or jaundice and to contact their healthcare provider as soon as possible [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Lactation
Advise nursing women to avoid breastfeeding during treatment with sulfamethoxazole and trimethoprim.
Antibacterial Resistance
Counsel patients that antibacterial drugs including sulfamethoxazole and trimethoprim should only be used to treat bacterial infections. It does not treat viral infections (e.g., the common cold). Instruct patients to maintain an adequate fluid intake in order to prevent crystalluria and stone formation.
Diarrhea
Advise patients that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.Manufactured by:
Mylan Laboratories Limited
Bangalore, India1035464
-
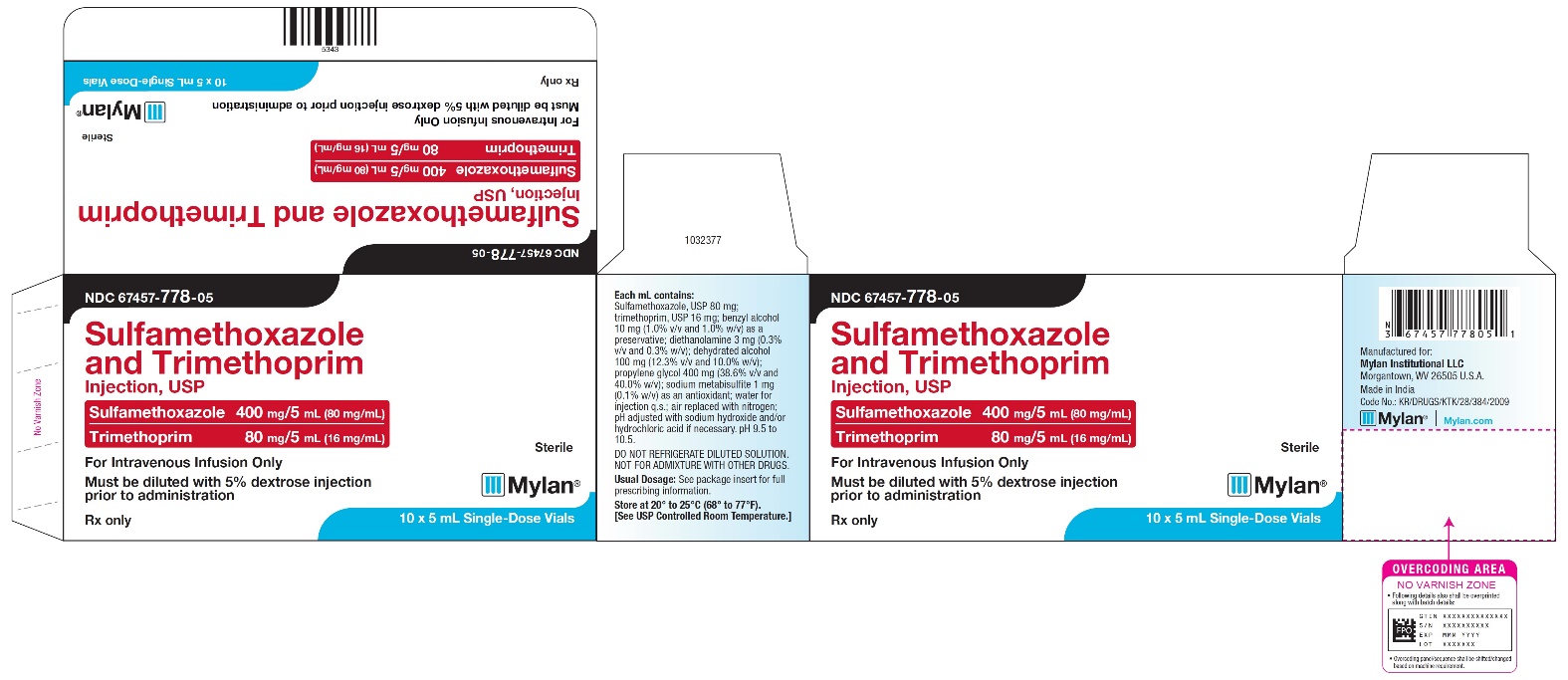
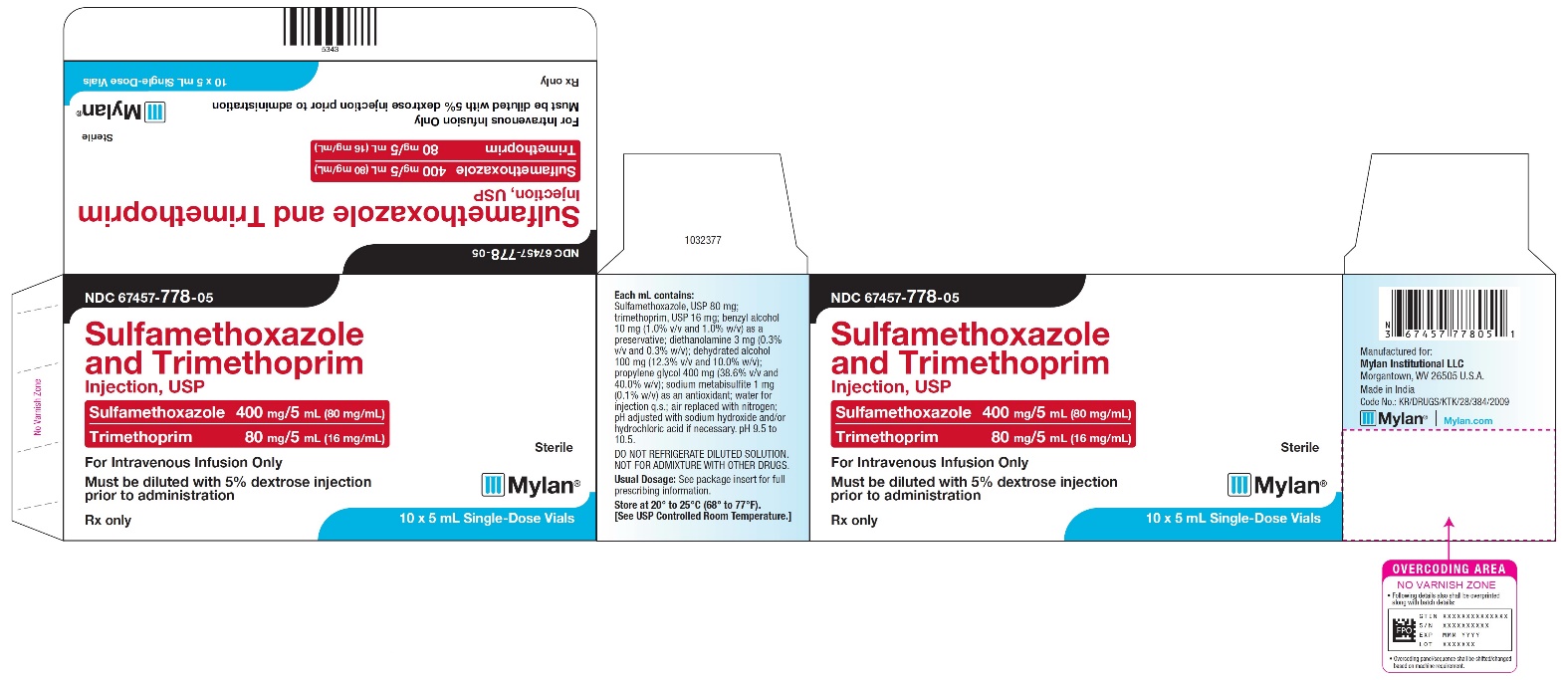
PRINCIPAL DISPLAY PANEL - NDC 67457-778-05
NDC 67457-778-05
Sulfamethoxazole
and Trimethoprim Injection, USPSulfamethoxazole 400 mg/5mL (80 mg/mL)
Trimethoprim 80 mg/5 mL (16 mg/mL)For Intravenous Infusion Only
Must be diluted with 5% dextrose injection
prior to administrationSterile
Mylan
Rx only
10 x 5 mL Single-Dose Vials
Each mL contains:
Sulfamethoxazole, USP 80 mg;
trimethoprim, USP 16 mg; benzyl alcohol
10 mg (1.0% v/v and 1.0% w/v) as a
preservative; diethanolamine 3 mg (0.3%
v/v and 0.3% w/v); dehydrated alcohol
100 mg (12.3% v/v and 10.0% w/v);
propylene glycol 400 mg (38.6% v/v and
40.0% w/v); sodium metabisulfite 1 mg
(0.1% w/v) as an antioxidant; water for
injection q.s.; air replaced with nitrogen;
pH adjusted with sodium hydroxide and/or
hydrochloric acid if necessary. pH 9.5 to
10.5.DO NOT REFRIGERATE DILUTED SOLUTION.
NOT FOR ADMIXTURE WITH OTHER DRUGS.Usual Dosage: See package insert for full
prescribing information.Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]Manufactured for: Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.Made in India
Code No.: KR/DRUGS/KTK/28/384/2009
Mylan.com
-
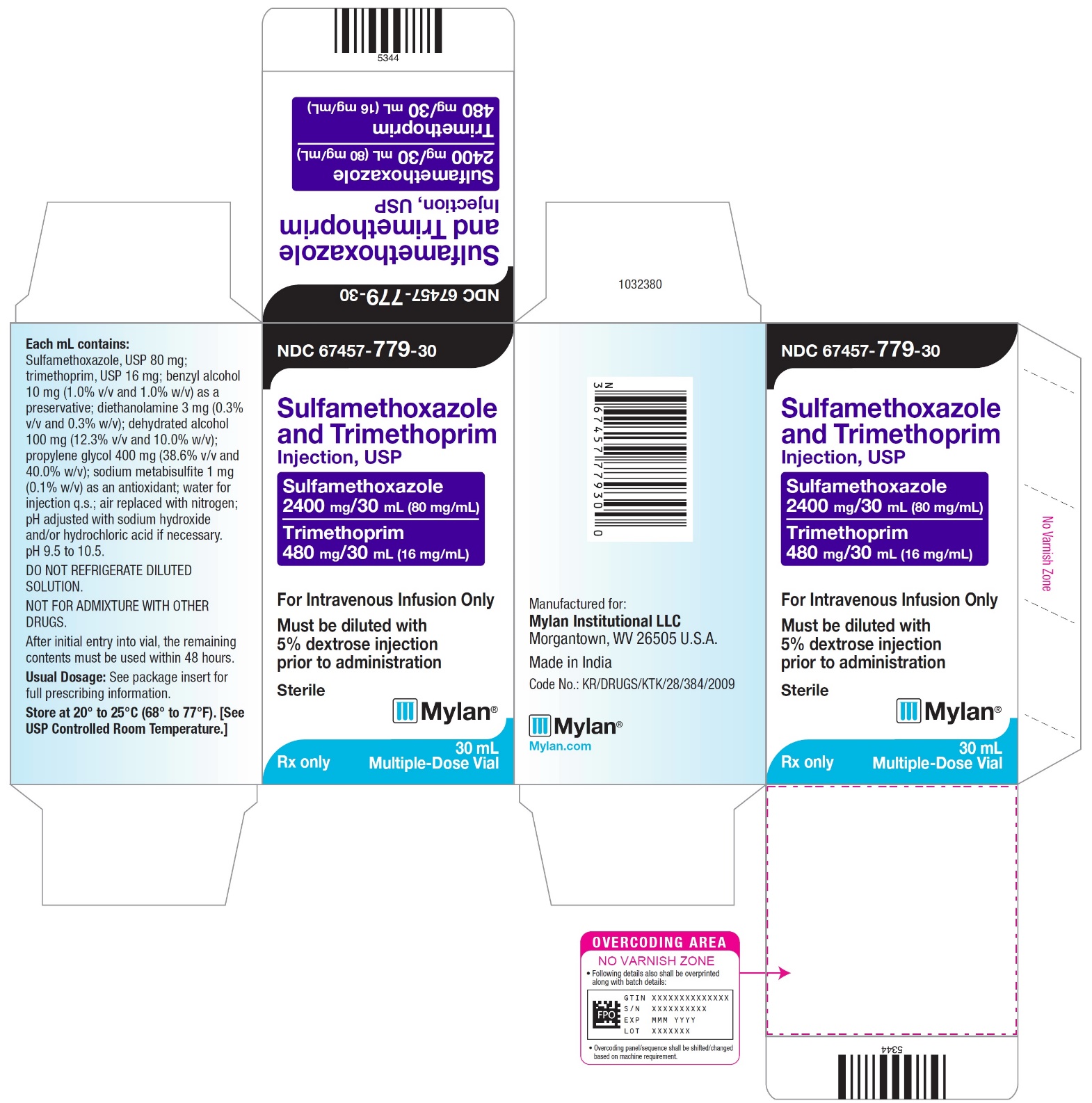
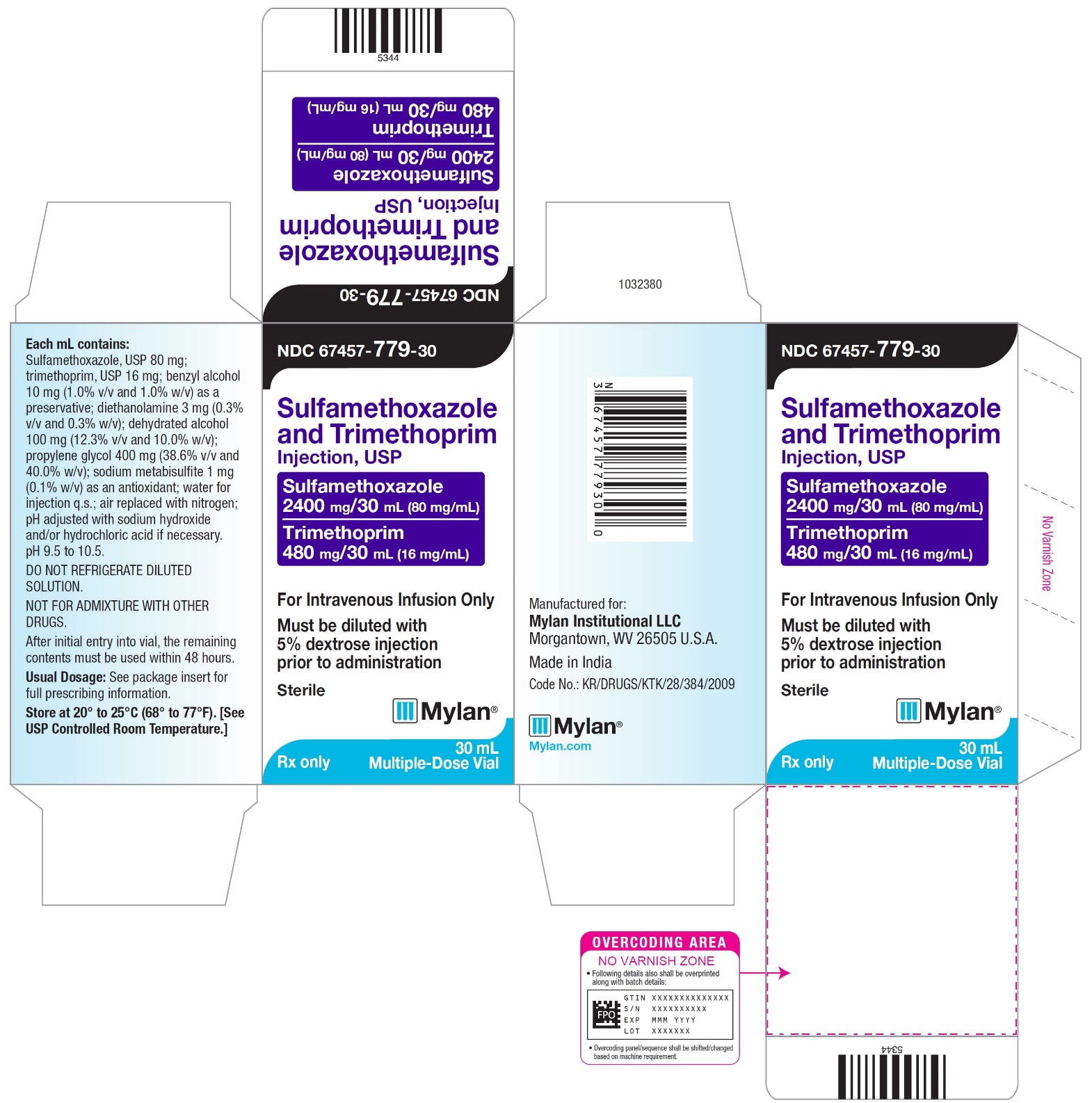
PRINCIPAL DISPLAY PANEL - NDC 67457-779-30
NDC 67457-779-30
Sulfamethoxazole
and Trimethoprim
Injection, USPSulfamethoxazole
400 mg/5mL (80 mg/mL)Trimethoprim
80 mg/5 mL (16 mg/mL)For Intravenous Infusion Only
Must be diluted with
5% dextrose injection
prior to administrationSterile
Mylan
Rx only
30 mL Multiple-Dose Vials
Each mL contains:
Sulfamethoxazole, USP 80 mg;
trimethoprim, USP 16 mg; benzyl alcohol
10 mg (1.0% v/v and 1.0% w/v) as a
preservative; diethanolamine 3 mg (0.3%
v/v and 0.3% w/v); dehydrated alcohol
100 mg (12.3% v/v and 10.0% w/v);
propylene glycol 400 mg (38.6% v/v and
40.0% w/v); sodium metabisulfite 1 mg
(0.1% w/v) as an antioxidant; water for
injection q.s.; air replaced with nitrogen;
pH adjusted with sodium hydroxide
and/or hydrochloric acid if necessary.
pH 9.5 to 10.5.DO NOT REFRIGERATE DILUTED
SOLUTION.NOT FOR ADMIXTURE WITH OTHER
DRUGS.After initial entry into vial, the remaining
contents must be used within 48 hours.Usual Dosage: See package insert for
full prescribing information.Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.Made in India
Code No.: KR/DRUGS/KTK/28/384/2009
Mylan.com
-
INGREDIENTS AND APPEARANCE
SULFAMETHOXAZOLE AND TRIMETHOPRIM
sulfamethoxazole and trimethoprim injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67457-778 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFAMETHOXAZOLE (UNII: JE42381TNV) (SULFAMETHOXAZOLE - UNII:JE42381TNV) SULFAMETHOXAZOLE 80 mg in 1 mL TRIMETHOPRIM (UNII: AN164J8Y0X) (TRIMETHOPRIM - UNII:AN164J8Y0X) TRIMETHOPRIM 16 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) DIETHANOLAMINE (UNII: AZE05TDV2V) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) NITROGEN (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67457-778-05 10 in 1 CARTON 12/31/2017 1 NDC:67457-778-00 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206607 12/31/2017 SULFAMETHOXAZOLE AND TRIMETHOPRIM
sulfamethoxazole and trimethoprim injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67457-779 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFAMETHOXAZOLE (UNII: JE42381TNV) (SULFAMETHOXAZOLE - UNII:JE42381TNV) SULFAMETHOXAZOLE 80 mg in 1 mL TRIMETHOPRIM (UNII: AN164J8Y0X) (TRIMETHOPRIM - UNII:AN164J8Y0X) TRIMETHOPRIM 16 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) DIETHANOLAMINE (UNII: AZE05TDV2V) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) NITROGEN (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67457-779-30 1 in 1 CARTON 12/31/2017 1 30 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206607 12/31/2017 Labeler - Mylan Institutional LLC (790384502)