Label: SILDENAFIL CITRATE- sildenafil tablet, film coated
- NDC Code(s): 43063-941-15
- Packager: PD-Rx Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 13668-187
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SILDENAFIL TABLETS safely and effectively. See full prescribing information for SILDENAFIL TABLETS. SILDENAFIL tablets, for oral ...These highlights do not include all the information needed to use SILDENAFIL TABLETS safely and effectively. See full prescribing information for SILDENAFIL TABLETS.
SILDENAFIL tablets, for oral use
Initial U.S. Approval:1998RECENT MAJOR CHANGES
Warnings and Precautions, Effects on the Eye ( 5.3) 08/2017
INDICATIONS AND USAGE
Sildenafil citrate is a phosphodiesterase-5 (PDE5) inhibitor indicated for the treatment of erectile dysfunction (ED). (1)
DOSAGE AND ADMINISTRATION
- For most patients, the recommended dose is 50 mg taken, as needed, approximately 1 hour before sexual activity. However, Sildenafil tablets may be taken anywhere from 30 minutes to 4 hours before sexual activity ( 2.1)
- Based on effectiveness and toleration, may increase to a maximum of 100 mg or decrease to 25 mg ( 2.1)
- Maximum recommended dosing frequency is once per day ( 2.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 25 mg, 50 mg and 100 mg ( 3)
CONTRAINDICATIONS
- Administration of sildenafil tablets to patients using nitric oxide donors, such as organic nitrates or organic nitrites in any form. Sildenafil citrate was shown to potentiate the hypotensive effect of nitrates ( 4.1, 7.1, 12.2)
- Known hypersensitivity to sildenafil or any component of tablet ( 4.2)
- Administration with guanylate cyclase (GC) stimulators, such as riociguat ( 4.3)
WARNINGS AND PRECAUTIONS
- Patients should not use sildenafil citrate if sexual activity is inadvisable due to cardiovascular status ( 5.1)
- Patients should seek emergency treatment if an erection lasts >4 hours. Use sildenafil citrate with caution in patients predisposed to priapism ( 5.2)
- Patients should stop sildenafil tablets and seek medical care if a sudden loss of vision occurs in one or both eyes, which could be a sign of non arteritic anterior ischemic optic neuropathy (NAION). Sildenafil tablets should be used with caution, and only when the anticipated benefits outweigh the risks, in patients with a history of NAION. Patients with a” crowded” optic disc may also be at an increased risk of NAION. ( 5.3)
- Patients should stop sildenafil tablets and seek prompt medical attention in the event of sudden decrease or loss of hearing ( 5.4)
- Caution is advised when Sildenafil citrate is co-administered with alpha- blockers or anti-hypertensives. Concomitant use may lead to hypotension ( 5.5)
- Decreased blood pressure, syncope, and prolonged erection may occur at higher sildenafil exposures. In patients taking strong CYP inhibitors, such as ritonavir, sildenafil exposure is increased. Decrease in Sildenafil citrate dosage is recommended ( 2.4, 5.6)
ADVERSE REACTIONS
Most common adverse reactions (≥ 2%) include headache, flushing, dyspepsia, abnormal vision, nasal congestion, back pain, myalgia, nausea, dizziness and rash ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Torrent Pharma Inc. at 1-800-912-9561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Sildenafil citrate can potentiate the hypotensive effects of nitrates, alpha blockers, and anti-hypertensives ( 4.1, 5.5, 7.1, 7.2, 7.3, 12.2)
- With concomitant use of alpha blockers, initiate sildenafil citrate at 25 mg dose ( 2.3)
- CYP3A4 inhibitors (e.g., ritonavir, ketoconazole, itraconazole, erythromycin): Increase Sildenafil citrate exposure ( 2.4, 7.4, 12.3)
- Ritonavir: Do not exceed a maximum single dose of 25 mg in a 48 hour period ( 2.4, 5.6)
- Erythromycin or strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, saquinavir): Consider a starting dose of 25 mg ( 2.4, 7.4)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
2.2 Use with Food
2.3 Dosage Adjustments in Specific Situations
2.4 Dosage Adjustments Due to Drug Interactions
2.5 Dosage Adjustments in Special Populations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Nitrates
4.2 Hypersensitivity Reactions
4.3 Concomitant Guanylate Cyclase (GC) Stimulators
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular
5.2 Prolonged Erection and Priapism
5.3 Effects on the Eye
5.4 Hearing Loss
5.5 Hypotension when Co-administered with Alpha-blockers or Anti-hypertensives
5.6 Adverse Reactions with the Concomitant Use of Ritonavir
5.7 Combination with other PDE5 Inhibitors or Other Erectile Dysfunction Therapies
5.8 Effects on Bleeding
5.9 Counseling Patients About Sexually Transmitted Diseases
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Nitrates
7.2 Alpha-blockers
7.3 Amlodipine
7.4 Ritonavir and other CYP3A4 inhibitors
7.5 Alcohol
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Sildenafil tablets are indicated for the treatment of erectile dysfunction.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information - For most patients, the recommended dose is 50 mg taken, as needed, approximately 1 hour before sexual activity. However, Sildenafil tablets may be taken anywhere from ...
2.1 Dosage Information
For most patients, the recommended dose is 50 mg taken, as needed, approximately 1 hour before sexual activity. However, Sildenafil tablets may be taken anywhere from 30 minutes to 4 hours before sexual activity. The maximum recommended dosing frequency is once per day.
Based on effectiveness and toleration, the dose may be increased to a maximum recommended dose of 100 mg or decreased to 25 mg.
2.3 Dosage Adjustments in Specific Situations
Sildenafil tablets was shown to potentiate the hypotensive effects of nitrates and its administration in patients who use nitric oxide donors such as organic nitrates or organic nitrites in any form is therefore contraindicated [see Contraindications (4.1), Drug Interactions (7.1), and Clinical Pharmacology (12.2)] .
When sildenafil tablets are co-administered with an alpha-blocker, patients should be stable on alpha-blocker therapy prior to initiating sildenafil citrate treatment and sildenafil citrate should be initiated at 25 mg [see Warnings and Precautions (5.5), Drug Interactions (7.2), and Clinical Pharmacology (12.2)] .
2.4 Dosage Adjustments Due to Drug Interactions
Ritonavir
The recommended dose for ritonavir-treated patients is 25 mg prior to sexual activity and the recommended maximum dose is 25 mg within a 48 hour period because concomitant administration increased the blood levels of sildenafil by 11-fold [see Warnings and Precautions (5.6), Drug Interactions (7.4), and Clinical Pharmacology (12.3)] .
CYP3A4 Inhibitors
Consider a starting dose of 25 mg in patients treated with strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, or saquinavir) or erythromycin. Clinical data have shown that co-administration with saquinavir or erythromycin increased plasma levels of sildenafil by about 3 fold [see Drug Interactions (7.4)and Clinical Pharmacology (12.3)] .
Close2.5 Dosage Adjustments in Special Populations
Consider a starting dose of 25 mg in patients > 65 years, patients with hepatic impairment (e.g., cirrhosis), and patients with severe renal impairment (creatinine clearance <30 mL/minute) because administration of sildenafil tablets in these patients resulted in higher plasma levels of sildenafil [ see Use in Specific Populations ( 8.5, 8.6, 8.7) and Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
Sildenafil tablets USP are supplied as blue, round, biconvex, film-coated tablets containing sildenafil citrate equivalent to 25 mg, 50 mg or 100 mg of sildenafil. Tablets are debossed with 86, 87 ...
Sildenafil tablets USP are supplied as blue, round, biconvex, film-coated tablets containing sildenafil citrate equivalent to 25 mg, 50 mg or 100 mg of sildenafil. Tablets are debossed with 86, 87 and 88 respectively for 25 mg, 50 mg and 100 mg strength on one side and plain on other side.
Close -
4 CONTRAINDICATIONS
4.1 Nitrates - Consistent with its known effects on the nitric oxide/cGMP pathway [ see Clinical Pharmacology ( 12.1, 12.2) ], sildenafil citrate was shown to potentiate the ...
4.1 Nitrates
Consistent with its known effects on the nitric oxide/cGMP pathway [ see Clinical Pharmacology ( 12.1, 12.2) ], sildenafil citrate was shown to potentiate the hypotensive effects of nitrates, and its administration to patients who are using nitric oxide donors such as organic nitrates or organic nitrites in any form either regularly and/or intermittently is therefore contraindicated.
After patients have taken sildenafil citrate, it is unknown when nitrates, if necessary, can be safely administered. Although plasma levels of sildenafil at 24 hours post dose are much lower than at peak concentration, it is unknown whether nitrates can be safely co-administered at this time point [ see Dosage and Administration (2.3), Drug Interactions (7.1), and Clinical Pharmacology (12.2)].
4.2 Hypersensitivity Reactions
Sildenafil tablets is contraindicated in patients with a known hypersensitivity to sildenafil, as contained in sildenafil tablets, 25 mg, 50 mg and 100 mg and sildenafil tablets, 20 mg, or any component of the tablet. Hypersensitivity reactions have been reported, including rash and urticaria [ see Adverse Reactions (6.1)].
Close4.3 Concomitant Guanylate Cyclase (GC) Stimulators
Do not use sildenafil tablets in patients who are using a GC stimulator, such as riociguat. PDE5 inhibitors, including sildenafil citrate, may potentiate the hypotensive effects of GC stimulators.
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular - There is a potential for cardiac risk of sexual activity in patients with preexisting cardiovascular disease. Therefore, treatments for erectile dysfunction, including ...
5.1 Cardiovascular
There is a potential for cardiac risk of sexual activity in patients with preexisting cardiovascular disease. Therefore, treatments for erectile dysfunction, including sildenafil citrate, should not be generally used in men for whom sexual activity is inadvisable because of their underlying cardiovascular status. The evaluation of erectile dysfunction should include a determination of potential underlying causes and the identification of appropriate treatment following a complete medical assessment.
Sildenafil citrate has systemic vasodilatory properties that resulted in transient decreases in supine blood pressure in healthy volunteers (mean maximum decrease of 8.4/5.5 mmHg), [ see Clinical Pharmacology (12.2)]. While this normally would be expected to be of little consequence in most patients, prior to prescribing sildenafil citrate, physicians should carefully consider whether their patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects, especially in combination with sexual activity.
Use with caution in patients with the following underlying conditions which can be particularly sensitive to the actions of vasodilators including sildenafil citrate – those with left ventricular outflow obstruction (e.g., aortic stenosis, idiopathic hypertrophic subaortic stenosis) and those with severely impaired autonomic control of blood pressure.
There are no controlled clinical data on the safety or efficacy of sildenafil citrate in the following groups; if prescribed, this should be done with caution.
- Patients who have suffered a myocardial infarction, stroke, or life-threatening arrhythmia within the last 6 months;
- Patients with resting hypotension (BP <90/50 mmHg) or hypertension (BP >170/110 mmHg);
- Patients with cardiac failure or coronary artery disease causing unstable angina.
5.2 Prolonged Erection and Priapism
Prolonged erection greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been reported infrequently since market approval of sildenafil tablets. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency could result.
Sildenafil citrate should be used with caution in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie’s disease), or in patients who have conditions which may predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia). However, there are no controlled clinical data on the safety or efficacy of sildenafil citrate in patients with sickle cell or related anemias.
5.3 Effects on the Eye
Physicians should advise patients to stop use of all phosphodiesterase type 5 (PDE5) inhibitors, including sildenafil citrate, and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition and a cause of decreased vision including permanent loss of vision, that has been reported rarely post-marketing in temporal association with the use of all PDE5 inhibitors. Based on published literature, the annual incidence of NAION is 2.5 to 11.8 cases per 100,000 in males aged ≥ 50. An observational case-crossover study evaluated the risk of NAION when PDE5 inhibitor use, as a class, occurred immediately before NAION onset (within 5 half-lives), compared to PDE5 inhibitor use in a prior time period. The results suggest an approximate 2-fold increase in the risk of NAION, with a risk estimate of 2.15 (95% CI 1.06, 4.34). A similar study reported a consistent result, with a risk estimate of 2.27 (95% CI 0.99, 5.20). Other risk factors for NAION, such as the presence of “crowded” optic disc, may have contributed to the occurrence of NAION in these studies.
Neither the rare post-marketing reports, nor the association of PDE5 inhibitor use and NAION in the observational studies, substantiate a causal relationship between PDE5 inhibitor use and NAION [ see Adverse Reactions (6.2)].
Physicians should consider whether their patients with underlying NAION risk factors could be adversely affected by use of PDE5 inhibitors. Individuals who have already experienced NAION are at increased risk of NAION recurrence. Therefore, PDE5 inhibitors, including sildenafil citrate, should be used with caution in these patients and only when the anticipated benefits outweigh the risks. Individuals with “crowded” optic disc are also considered at greater risk for NAION compared to the general population, however, evidence is insufficient to support screening of prospective users of PDE5 inhibitors, including sildenafil citrate, for this uncommon condition.
There are no controlled clinical data on the safety or efficacy of sildenafil citrate in patients with retinitis pigmentosa (a minority of these patients have genetic disorders of retinal phosphodiesterases); if prescribed, this should be done with caution.
5.4 Hearing Loss
Physicians should advise patients to stop taking PDE5 inhibitors, including sildenafil citrate, and seek prompt medical attention in the event of sudden decrease or loss of hearing. These events, which may be accompanied by tinnitus and dizziness, have been reported in temporal association to the intake of PDE5 inhibitors, including sildenafil citrate. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [ see Adverse Reactions ( 6.1, 6.2) ].
5.5 Hypotension when Co-administered with Alpha-blockers or Anti-hypertensives
Alpha-blockers
Caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. PDE5 inhibitors, including sildenafil citrate, and alpha-adrenergic blocking agents are both vasodilators with blood pressure lowering effects.
When vasodilators are used in combination, an additive effect on blood pressure may occur. In some patients, concomitant use of these two drug classes can lower blood pressure significantly [ see Drug Interactions (7.2)and Clinical Pharmacology (12.2)] leading to symptomatic hypotension (e.g., dizziness, lightheadedness, fainting).
Consideration should be given to the following:
- Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant use of PDE5 inhibitors. Patients should be stable on alpha-blocker therapy prior to initiating a PDE5 inhibitor.
- In those patients who are stable on alpha-blocker therapy, PDE5 inhibitors should be initiated at the lowest dose [ see Dosage and Administration (2.3)].
- In those patients already taking an optimized dose of a PDE5 inhibitor, alpha-blocker therapy should be initiated at the lowest dose. Stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure when taking a PDE5 inhibitor.
- Safety of combined use of PDE5 inhibitors and alpha-blockers may be affected by other variables, including intravascular volume depletion and other anti-hypertensive drugs.
Anti-hypertensives
Sildenafil citrate has systemic vasodilatory properties and may further lower blood pressure in patients taking anti-hypertensive medications.
In a separate drug interaction study, when amlodipine, 5 mg or 10 mg, and sildenafil citrate, 100 mg were orally administered concomitantly to hypertensive patients mean additional blood pressure reduction of 8 mmHg systolic and 7 mmHg diastolic were noted [ see Drug Interactions (7.3)and Clinical Pharmacology (12.2)].
5.6 Adverse Reactions with the Concomitant Use of Ritonavir
The concomitant administration of the protease inhibitor ritonavir substantially increases serum concentrations of sildenafil (11-fold increase in AUC). If sildenafil citrate is prescribed to patients taking ritonavir, caution should be used. Data from subjects exposed to high systemic levels of sildenafil are limited. Decreased blood pressure, syncope, and prolonged erection were reported in some healthy volunteers exposed to high doses of sildenafil (200 to 800 mg). To decrease the chance of adverse reactions in patients taking ritonavir, a decrease in sildenafil dosage is recommended [ see Dosage and Administration (2.4), Drug Interactions (7.4), and Clinical Pharmacology (12.3)].
5.7 Combination with other PDE5 Inhibitors or Other Erectile Dysfunction Therapies
The safety and efficacy of combinations of sildenafil tablets, 25 mg, 50 mg and 100 mg with other PDE5 Inhibitors, including sildenafil tablets, 20 mg or other pulmonary arterial hypertension (PAH) treatments containing sildenafil, or other treatments for erectile dysfunction have not been studied. Such combinations may further lower blood pressure. Therefore, the use of such combinations is not recommended.
5.8 Effects on Bleeding
There have been postmarketing reports of bleeding events in patients who have taken sildenafil citrate. A causal relationship between sildenafil citrate and these events has not been established. In humans, sildenafil citrate has no effect on bleeding time when taken alone or with aspirin. However, in vitro studies with human platelets indicate that sildenafil potentiates the antiaggregatory effect of sodium nitroprusside (a nitric oxide donor). In addition, the combination of heparin and sildenafil citrate had an additive effect on bleeding time in the anesthetized rabbit, but this interaction has not been studied in humans.
The safety of sildenafil citrate is unknown in patients with bleeding disorders and patients with active peptic ulceration.
Close5.9 Counseling Patients About Sexually Transmitted Diseases
The use of sildenafil citrate offers no protection against sexually transmitted diseases. Counseling of patients about the protective measures necessary to guard against sexually transmitted diseases, including the Human Immunodeficiency Virus (HIV), may be considered.
-
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling: Cardiovascular [ see - Warnings and Precautions (5.1)] Prolonged Erection and Priapism [ see ...
The following are discussed in more detail in other sections of the labeling:
- Cardiovascular [ see Warnings and Precautions (5.1)]
- Prolonged Erection and Priapism [ see Warnings and Precautions (5.2)]
- Effects on the Eye [ see Warnings and Precautions (5.3)]
- Hearing Loss [ see Warnings and Precautions (5.4)]
- Hypotension when Co-administered with Alpha-blockers or Anti-hypertensives [ see Warnings and Precautions (5.5)]
- Adverse Reactions with the Concomitant Use of Ritonavir [ see Warnings and Precautions (5.6)]
- Combination with other PDE5 Inhibitors or Other Erectile Dysfunction Therapies [ see Warnings and Precautions (5.7)]
- Effects on Bleeding [ see Warnings and Precautions (5.8)]
- Counseling Patients About Sexually Transmitted Diseases [ see Warnings and Precautions (5.9)]
The most common adverse reactions reported in clinical trials (≥ 2%) are headache, flushing, dyspepsia, abnormal vision, nasal congestion, back pain, myalgia, nausea, dizziness, and rash.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Sildenafil citrate was administered to over 3,700 patients (aged 19 to 87 years) during pre-marketing clinical trials worldwide. Over 550 patients were treated for longer than one year.
In placebo-controlled clinical studies, the discontinuation rate due to adverse reactions for sildenafil citrate (2.5%) was not significantly different from placebo (2.3%).
In fixed-dose studies, the incidence of some adverse reactions increased with dose. The type of adverse reactions in flexible-dose studies, which reflect the recommended dosage regimen, was similar to that for fixed-dose studies. At doses above the recommended dose range, adverse reactions were similar to those detailed in Table 1 below but generally were reported more frequently.
Table 1. Adverse Reactions Reported by ≥2% of Patients Treated with Sildenafil Citrate and More Frequent than Placebo in Fixed-Dose Phase II/III Studies Adverse Reaction 25 mg
(n=312)50 mg
(n=511)100 mg
(n=506)Placebo
(n=607)Headache 16% 21% 28% 7% Flushing 10% 19% 18% 2% Dyspepsia 3% 9% 17% 2% Abnormal vision † 1% 2% 11% 1% Nasal congestion 4% 4% 9% 2% Back pain 3% 4% 4% 2% Myalgia 2% 2% 4% 1% Nausea 2% 3% 3% 1% Dizziness 3% 4% 3% 2% Rash 1% 2% 3% 1% †Abnormal Vision: Mild to moderate in severity and transient, predominantly color tinge to vision, but also increased sensitivity to light, or blurred vision.
When sildenafil citrate was taken as recommended (on an as-needed basis) in flexible-dose, placebo-controlled clinical trials of two to twenty-six weeks duration, patients took sildenafil citrate at least once weekly, and the following adverse reactions were reported:
Table 2. Adverse Reactions Reported by ≥2% of Patients Treated with Sildenafil Citrate and More Frequent than Placebo in Flexible-Dose Phase II/III Studies Adverse Reaction SILDENAFIL CITRATE PLACEBO N=734 N=725 Headache 16% 4% Flushing 10% 1% Dyspepsia 7% 2% Nasal Congestion 4% 2% Abnormal Vision † 3% 0% Back pain 2% 2% Dizziness 2% 1% Rash 2% 1% †Abnormal Vision: Mild and transient, predominantly color tinge to vision, but also increased sensitivity to light, or blurred vision. In these studies, only one patient discontinued due to abnormal vision.
The following events occurred in <2% of patients in controlled clinical trials; a causal relationship to sildenafil citrate is uncertain. Reported events include those with a plausible relation to drug use; omitted are minor events and reports too imprecise to be meaningful:
Body as a Whole:face edema, photosensitivity reaction, shock, asthenia, pain, chills, accidental fall, abdominal pain, allergic reaction, chest pain, accidental injury.
Cardiovascular:angina pectoris, AV block, migraine, syncope, tachycardia, palpitation, hypotension, postural hypotension, myocardial ischemia, cerebral thrombosis, cardiac arrest, heart failure, abnormal electrocardiogram, cardiomyopathy.
Digestive:vomiting, glossitis, colitis, dysphagia, gastritis, gastroenteritis, esophagitis, stomatitis, dry mouth, liver function tests abnormal, rectal hemorrhage, gingivitis.
Hemic and Lymphatic:anemia and leukopenia.
Metabolic and Nutritional:thirst, edema, gout, unstable diabetes, hyperglycemia, peripheral edema, hyperuricemia, hypoglycemic reaction, hypernatremia.
Musculoskeletal:arthritis, arthrosis, myalgia, tendon rupture, tenosynovitis, bone pain, myasthenia, synovitis.
Nervous:ataxia, hypertonia, neuralgia, neuropathy, paresthesia, tremor, vertigo, depression, insomnia, somnolence, abnormal dreams, reflexes decreased, hypesthesia.
Respiratory:asthma, dyspnea, laryngitis, pharyngitis, sinusitis, bronchitis, sputum increased, cough increased.
Skin and Appendages:urticaria, herpes simplex, pruritus, sweating, skin ulcer, contact dermatitis, exfoliative dermatitis.
Special Senses:sudden decrease or loss of hearing, mydriasis, conjunctivitis, photophobia, tinnitus, eye pain, ear pain, eye hemorrhage, cataract, dry eyes.
Urogenital:cystitis, nocturia, urinary frequency, breast enlargement, urinary incontinence, abnormal ejaculation, genital edema and anorgasmia.
Analysis of the safety database from controlled clinical trials showed no apparent difference in adverse reactions in patients taking sildenafil citrate with and without anti-hypertensive medication. This analysis was performed retrospectively, and was not powered to detect any pre-specified difference in adverse reactions.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of sildenafil citrate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion either due to their seriousness, reporting frequency, lack of clear alternative causation, or a combination of these factors.
Cardiovascular and cerebrovascular
Serious cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death, ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, subarachnoid and intracerebral hemorrhages, and pulmonary hemorrhage have been reported post-marketing in temporal association with the use of sildenafil citrate. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of sildenafil citrate without sexual activity. Others were reported to have occurred hours to days after the use of sildenafil citrate and sexual activity. It is not possible to determine whether these events are related directly to sildenafil citrate, to sexual activity, to the patient’s underlying cardiovascular disease, to a combination of these factors, or to other factors [ see Warnings and Precautions (5.1)and Patient Counseling Information (17)].
Hemic and Lymphatic:vaso-occlusive crisis: In a small, prematurely terminated study of sildenafil tablets, 20 mg (sildenafil) in patients with pulmonary arterial hypertension (PAH) secondary to sickle cell disease, vaso-occlusive crises requiring hospitalization were more commonly reported in patients who received sildenafil than in those randomized to placebo. The clinical relevance of this finding to men treated with sildenafil tablets, 25 mg, 50 mg and 100 mg for ED is not known.
Nervous:seizure, seizure recurrence, anxiety, and transient global amnesia.
Respiratory:epistaxis
Special senses:
Hearing:Cases of sudden decrease or loss of hearing have been reported postmarketing in temporal association with the use of PDE5 inhibitors, including sildenafil citrate. In some of the cases, medical conditions and other factors were reported that may have also played a role in the otologic adverse events. In many cases, medical follow-up information was limited. It is not possible to determine whether these reported events are related directly to the use of sildenafil citrate, to the patient’s underlying risk factors for hearing loss, a combination of these factors, or to other factors [ see Warnings and Precautions (5.4)and Patient Counseling Information (17)].
Ocular:diplopia, temporary vision loss/decreased vision, ocular redness or bloodshot appearance, ocular burning, ocular swelling/pressure, increased intraocular pressure, retinal edema, retinal vascular disease or bleeding, and vitreous traction/detachment.
Non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported rarely post-marketing in temporal association with the use of phosphodiesterase type 5 (PDE5) inhibitors, including sildenafil citrate. Most, but not all, of these patients had underlying anatomic or vascular risk factors for developing NAION, including but not necessarily limited to: low cup to disc ratio (“crowded disc”), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia and smoking [ see Warnings and Precautions (5.3)and Patient Counseling Information (17)].
Urogenital:prolonged erection, priapism [ see Warnings and Precautions (5.2)and Patient Counseling Information (17)], and hematuria.
-
7 DRUG INTERACTIONS
7.1 Nitrates - Administration of sildenafil citrate with nitric oxide donors such as organic nitrates or organic nitrites in any form is contraindicated. Consistent with its known effects on ...
7.1 Nitrates
Administration of sildenafil citrate with nitric oxide donors such as organic nitrates or organic nitrites in any form is contraindicated. Consistent with its known effects on the nitric oxide/cGMP pathway, sildenafil citrate was shown to potentiate the hypotensive effects of nitrates [ see Dosage and Administration (2.3), Contraindications (4.1), Clinical Pharmacology (12.2)].
7.2 Alpha-blockers
Use caution when co-administering alpha-blockers with sildenafil citrate because of potential additive blood pressure-lowering effects. When sildenafil citrate is co-administered with an alpha-blocker, patients should be stable on alpha-blocker therapy prior to initiating sildenafil citrate treatment and sildenafil citrate should be initiated at the lowest dose [ see Dosage and Administration (2.3), Warnings and Precautions (5.5), Clinical Pharmacology (12.2)].
7.3 Amlodipine
When sildenafil citrate 100 mg was co-administered with amlodipine (5 mg or 10 mg) to hypertensive patients, the mean additional reduction on supine blood pressure was 8 mmHg systolic and 7 mmHg diastolic [ see Warnings and Precautions (5.5), Clinical Pharmacology (12.2)].
7.4 Ritonavir and other CYP3A4 inhibitors
Co-administration of ritonavir, a strong CYP3A4 inhibitor, greatly increased the systemic exposure of sildenafil (11-fold increase in AUC). It is therefore recommended not to exceed a maximum single dose of 25 mg of sildenafil citrate in a 48 hour period [ see Dosage and Administration (2.4), Warnings and Precautions (5.6), Clinical Pharmacology (12.3)].
Co-administration of erythromycin, a moderate CYP3A4 inhibitor, resulted in a 160% and 182% increases in sildenafil C maxand AUC, respectively. Co-administration of saquinavir, a strong CYP3A4 inhibitor, resulted in 140% and 210% increases in sildenafil C maxand AUC, respectively. Stronger CYP3A4 inhibitors such as ketoconazole or itraconazole could be expected to have greater effects than seen with saquinavir. A starting dose of 25 mg of sildenafil citrate should be considered in patients taking erythromycin or strong CYP3A4 inhibitors (such as saquinavir, ketoconazole, itraconazole) [ see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
Close7.5 Alcohol
In a drug-drug interaction study sildenafil 50 mg given with alcohol 0.5 g/kg in which mean maximum blood alcohol levels of 0.08% was achieved, sildenafil did not potentiate the hypotensive effect of alcohol in healthy volunteers [see Clinical Pharmacology (12.2)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Sildenafil Citrate is not indicated for use in females. There are no data with the use of Sildenafil Citrate in pregnant women to inform any drug-associated ...
8.1 Pregnancy
Risk Summary
Sildenafil Citrate is not indicated for use in females.
There are no data with the use of Sildenafil Citrate in pregnant women to inform any drug-associated risks for adverse developmental outcomes. Animal reproduction studies conducted with sildenafil did not show adverse developmental outcomes when administered during organogenesis in rats and rabbits at oral doses up to 16 and 32 times, respectively, the maximum recommended human dose (MRHD) of 100 mg/day on a mg/m 2basis (see Data).
Data
Animal Data
No evidence of teratogenicity, embryotoxicity or fetotoxicity was observed in rats and rabbits which received oral doses up to 200 mg/kg/day during organogenesis. These doses represent, respectively, about 16 and 32 times the MRHD on a mg/m 2basis in a 50 kg subject. In the rat pre- and postnatal development study, the no observed adverse effect dose was 30 mg/kg/day given for 36 days, about 2 times the MRHD on a mg/m 2basis in a 50 kg subject.
8.4 Pediatric Use
Sildenafil citrate is not indicated for use in pediatric patients. Safety and effectiveness have not been established in pediatric patients.
8.5 Geriatric Use
Healthy elderly volunteers (65 years or over) had a reduced clearance of sildenafil resulting in approximately 84% and 107% higher plasma AUC values of sildenafil and its active N-desmethyl metabolite, respectively, compared to those seen in healthy young volunteers (18 to 45 years) [ see Clinical Pharmacology (12.3)]. Due to age-differences in plasma protein binding, the corresponding increase in the AUC of free (unbound) sildenafil and its active N-desmethyl metabolite were 45% and 57%, respectively [ see Clinical Pharmacology (12.3)].
Of the total number of subjects in clinical studies of sildenafil citrate, 18% were 65 years and older, while 2% were 75 years and older. No overall differences in safety or efficacy were observed between older (≥ 65 years of age) and younger (< 65 years of age) subjects.
However, since higher plasma levels may increase the incidence of adverse reactions, a starting dose of 25 mg should be considered in older subjects due to the higher systemic exposure [ see Dosage and Administration (2.5)].
8.6 Renal Impairment
No dose adjustment is required for mild (CLcr=50 to 80 mL/min) and moderate (CLcr=30 to 49 mL/min) renal impairment. In volunteers with severe renal impairment (Clcr<30 mL/min), sildenafil clearance was reduced, resulting in higher plasma exposure of sildenafil (~2 fold), approximately doubling of C maxand AUC. A starting dose of 25 mg should be considered in patients with severe renal impairment [ see Dosage and Administration (2.5)and Clinical Pharmacology (12.3)].
Close8.7 Hepatic Impairment
In volunteers with hepatic impairment (Child-Pugh Class A and B), sildenafil clearance was reduced, resulting in higher plasma exposure of sildenafil (47% for C maxand 85% for AUC). The pharmacokinetics of sildenafil in patients with severely impaired hepatic function (Child-Pugh Class C) have not been studied. A starting dose of 25 mg should be considered in patients with any degree of hepatic impairment [ see Dosage and Administration (2.5)and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In studies with healthy volunteers of single doses up to 800 mg, adverse reactions were similar to those seen at lower doses but incidence rates and severities were increased. In cases of ...
In studies with healthy volunteers of single doses up to 800 mg, adverse reactions were similar to those seen at lower doses but incidence rates and severities were increased.
In cases of overdose, standard supportive measures should be adopted as required. Renal dialysis is not expected to accelerate clearance as sildenafil is highly bound to plasma proteins and it is not eliminated in the urine.
Close -
11 DESCRIPTION
Sildenafil tablets USP, an oral therapy for erectile dysfunction, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type ...
Sildenafil tablets USP, an oral therapy for erectile dysfunction, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5).
Sildenafil citrate, USP is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1 H-pyrazolo [4,3- d] pyrimidin-5-yl)-4- ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate and has the following structural formula:
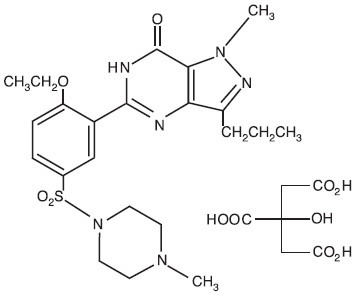
Sildenafil citrate, USP is a white to off-white crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7.
Sildenafil Citrate USP is formulated as blue, round, biconvex, film-coated tablets containing sildenafil citrate equivalent to 25 mg, 50 mg or 100 mg of sildenafil for oral administration. In addition to the active ingredient, sildenafil citrate, each tablet contains the following inactive ingredients: croscarmellose sodium, dibasic calcium phosphate anhydrous, hypromellose, lake of indigo carmine, microcrystalline cellulose, sodium stearyl fumarate, titanium dioxide and triacetin.
Close -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The physiologic mechanism of erection of the penis involves release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation. NO then activates the ...
12.1 Mechanism of Action
The physiologic mechanism of erection of the penis involves release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation. NO then activates the enzyme guanylate cyclase, which results in increased levels of cyclic guanosine monophosphate (cGMP), producing smooth muscle relaxation in the corpus cavernosum and allowing inflow of blood.
Sildenafil enhances the effect of NO by inhibiting phosphodiesterase type 5 (PDE5), which is responsible for degradation of cGMP in the corpus cavernosum. Sildenafil has no direct relaxant effect on isolated human corpus cavernosum. When sexual stimulation causes local release of NO, inhibition of PDE5 by sildenafil causes increased levels of cGMP in the corpus cavernosum, resulting in smooth muscle relaxation and inflow of blood to the corpus cavernosum. Sildenafil at recommended doses has no effect in the absence of sexual stimulation.
Binding Characteristics
Studies in vitrohave shown that sildenafil is selective for PDE5. Its effect is more potent on PDE5 than on other known phosphodiesterases (10-fold for PDE6, >80-fold for PDE1, >700-fold for PDE2, PDE3, PDE4, PDE7, PDE8, PDE9, PDE10, and PDE11). Sildenafil is approximately 4,000-fold more selective for PDE5 compared to PDE3. PDE3 is involved in control of cardiac contractility. Sildenafil is only about 10-fold as potent for PDE5 compared to PDE6, an enzyme found in the retina which is involved in the phototransduction pathway of the retina. This lower selectivity is thought to be the basis for abnormalities related to color vision [ see Clinical Pharmacology (12.2)].
In addition to human corpus cavernosum smooth muscle, PDE5 is also found in other tissues including platelets, vascular and visceral smooth muscle, and skeletal muscle, brain, heart, liver, kidney, lung, pancreas, prostate, bladder, testis, and seminal vesicle. The inhibition of PDE5 in some of these tissues by sildenafil may be the basis for the enhanced platelet antiaggregatory activity of NO observed in vitro, an inhibition of platelet thrombus formation in vivoand peripheral arterial-venous dilatation in vivo.
12.2 Pharmacodynamics
Effects of Sildenafil citrate on Erectile Response:In eight double-blind, placebo-controlled crossover studies of patients with either organic or psychogenic erectile dysfunction, sexual stimulation resulted in improved erections, as assessed by an objective measurement of hardness and duration of erections (RigiScan ®), after sildenafil citrate administration compared with placebo. Most studies assessed the efficacy of sildenafil citrate approximately 60 minutes post dose. The erectile response, as assessed by RigiScan ®, generally increased with increasing sildenafil dose and plasma concentration. The time course of effect was examined in one study, showing an effect for up to 4 hours but the response was diminished compared to 2 hours.
Effects of Sildenafil citrate on Blood Pressure:Single oral doses of sildenafil (100 mg) administered to healthy volunteers produced decreases in sitting blood pressure (mean maximum decrease in systolic/diastolic blood pressure of 8.3/5.3 mmHg). The decrease in sitting blood pressure was most notable approximately 1 to 2 hours after dosing, and was not different than placebo at 8 hours. Similar effects on blood pressure were noted with 25 mg, 50 mg and 100 mg of Sildenafil citrate, therefore the effects are not related to dose or plasma levels within this dosage range. Larger effects were recorded among patients receiving concomitant nitrates [ see Contraindications (4.1)].
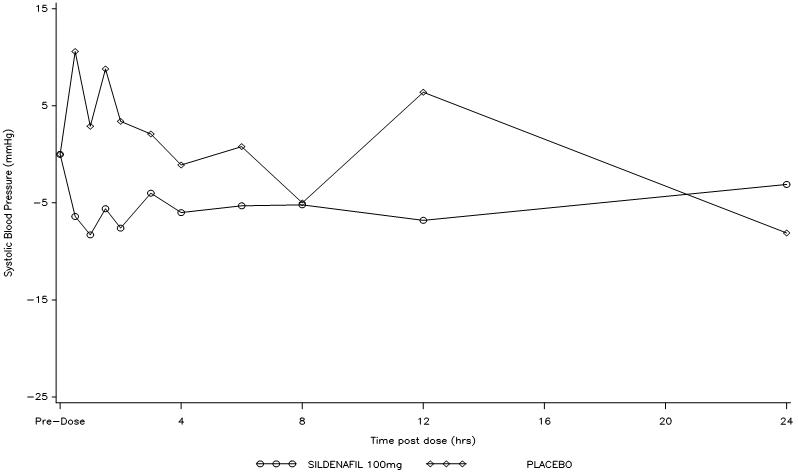
Figure 1: Mean Change from Baseline in Sitting Systolic Blood Pressure, Healthy Volunteers.
Effects of Sildenafil citrate on Blood Pressure When Nitroglycerin is Subsequently Administered:Based on the pharmacokinetic profile of a single 100 mg oral dose given to healthy normal volunteers, the plasma levels of sildenafil at 24 hours post dose are approximately 2 ng/mL (compared to peak plasma levels of approximately 440 ng/mL). In the following patients: age >65 years, hepatic impairment (e.g., cirrhosis), severe renal impairment (e.g., creatinine clearance <30 mL/min), and concomitant use of erythromycin or strong CYP3A4 inhibitors, plasma levels of sildenafil at 24 hours post dose have been found to be 3 to 8 times higher than those seen in healthy volunteers. Although plasma levels of sildenafil at 24 hours post dose are much lower than at peak concentration, it is unknown whether nitrates can be safely co-administered at this time point [ see Contraindications (4.1)].
Effects of Sildenafil citrate on Blood Pressure When Co-administered with Alpha-Blockers:Three double-blind, placebo-controlled, randomized, two-way crossover studies were conducted to assess the interaction of Sildenafil citrate with doxazosin, an alpha-adrenergic blocking agent.
Study 1: Sildenafil citrate with Doxazosin
In the first study, a single oral dose of Sildenafil citrate 100 mg or matching placebo was administered in a 2-period crossover design to 4 generally healthy males with benign prostatic hyperplasia (BPH). Following at least 14 consecutive daily doses of doxazosin, Sildenafil citrate 100 mg or matching placebo was administered simultaneously with doxazosin. Following a review of the data from these first 4 subjects (details provided below), the sildenafil citrate dose was reduced to 25 mg. Thereafter, 17 subjects were treated with Sildenafil citrate 25 mg or matching placebo in combination with doxazosin 4 mg (15 subjects) or doxazosin 8 mg (2 subjects). The mean subject age was 66.5 years.
For the 17 subjects who received sildenafil citrate 25 mg and matching placebo, the placebo-subtracted mean maximum decreases from baseline (95% CI) in systolic blood pressure were as follows:
Placebo-subtracted mean maximum decrease in systolic blood pressure (mm Hg) Sildenafil citrate 25 mg Supine 7.4 (-0.9 ,15.7) Standing 6.0 (-0.8, 12.8) The mean profiles of the change from baseline in standing systolic blood pressure in subjects treated with doxazosin in combination with 25 mg Sildenafil citrate or matching placebo are shown in Figure 2.
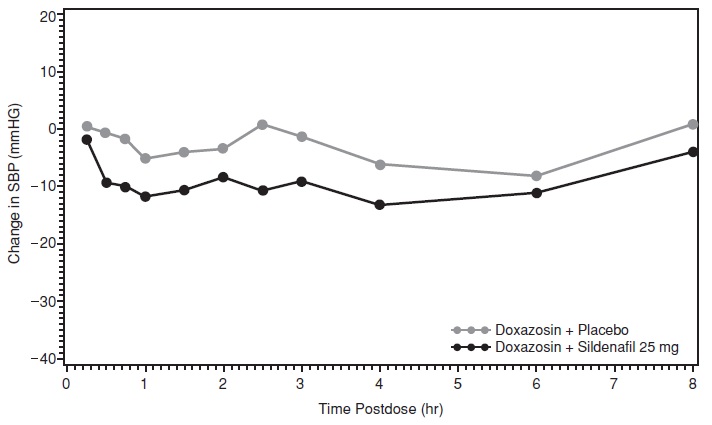
Figure 2: Mean Standing Systolic Blood Pressure Change from Baseline
Blood pressure was measured immediately pre-dose and at 15, 30, 45 minutes, and 1, 1.5, 2, 2.5, 3, 4, 6 and 8 hours after sildenafil citrate or matching placebo. Outliers were defined as subjects with a standing systolic blood pressure of <85 mmHg or a decrease from baseline in standing systolic blood pressure of >30 mmHg at one or more timepoints. There were no subjects treated with sildenafil citrate 25 mg who had a standing SBP < 85mmHg. There were three subjects with a decrease from baseline in standing systolic BP >30mmHg following sildenafil citrate 25 mg, one subject with a decrease from baseline in standing systolic BP > 30 mmHg following placebo and two subjects with a decrease from baseline in standing systolic BP > 30 mmHg following both sildenafil citrate and placebo. No severe adverse events potentially related to blood pressure effects were reported in this group.
Of the four subjects who received sildenafil citrate 100 mg in the first part of this study, a severe adverse event related to blood pressure effect was reported in one patient (postural hypotension that began 35 minutes after dosing with sildenafil citrate with symptoms lasting for 8 hours), and mild adverse events potentially related to blood pressure effects were reported in two others (dizziness, headache and fatigue at 1 hour after dosing; and dizziness, lightheadedness and nausea at 4 hours after dosing). There were no reports of syncope among these patients. For these four subjects, the placebo-subtracted mean maximum decreases from baseline in supine and standing systolic blood pressures were 14.8 mmHg and 21.5 mmHg, respectively. Two of these subjects had a standing SBP < 85mmHg. Both of these subjects were protocol violators, one due to a low baseline standing SBP, and the other due to baseline orthostatic hypotension.
Study 2: Sildenafil citrate with Doxazosin
In the second study, a single oral dose of sildenafil citrate 50 mg or matching placebo was administered in a 2-period crossover design to 20 generally healthy males with BPH. Following at least 14 consecutive days of doxazosin, sildenafil citrate 50 mg or matching placebo was administered simultaneously with doxazosin 4 mg (17 subjects) or with doxazosin 8 mg (3 subjects). The mean subject age in this study was 63.9 years.
Twenty subjects received sildenafil citrate 50 mg, but only 19 subjects received matching placebo. One patient discontinued the study prematurely due to an adverse event of hypotension following dosing with sildenafil citrate 50 mg. This patient had been taking minoxidil, a potent vasodilator, during the study.
For the 19 subjects who received both sildenafil citrate and matching placebo, the placebo-subtracted mean maximum decreases from baseline (95% CI) in systolic blood pressure were as follows:
Placebo-subtracted mean maximum decrease in systolic blood pressure (mm Hg) Sildenafil citrate 50 mg (95% CI) Supine 9.08 (5.48, 12.68) Standing 11.62 (7.34, 15.90) The mean profiles of the change from baseline in standing systolic blood pressure in subjects treated with doxazosin in combination with 50 mg sildenafil citrate or matching placebo are shown in Figure 3.
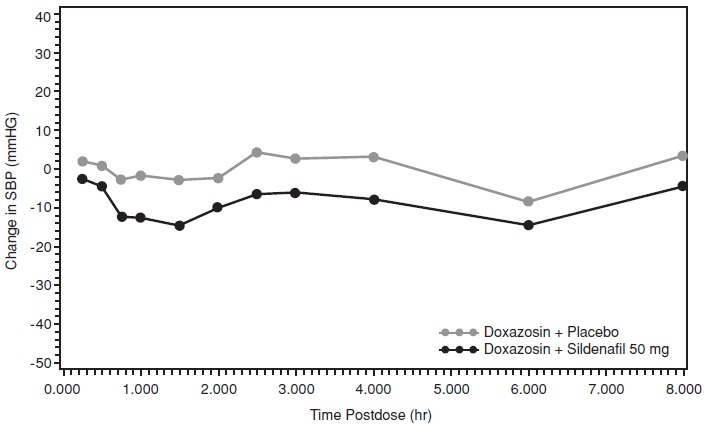
Figure 3: Mean Standing Systolic Blood Pressure Change from Baseline
Blood pressure was measured after administration of sildenafil citrate at the same times as those specified for the first doxazosin study. There were two subjects who had a standing SBP of < 85 mmHg. In these two subjects, hypotension was reported as a moderately severe adverse event, beginning at approximately 1 hour after administration of sildenafil citrate 50 mg and resolving after approximately 7.5 hours. There was one subject with a decrease from baseline in standing systolic BP >30mmHg following sildenafil citrate 50 mg and one subject with a decrease from baseline in standing systolic BP > 30 mmHg following both sildenafil citrate 50 mg and placebo. There were no severe adverse events potentially related to blood pressure and no episodes of syncope reported in this study.
Study 3: Sildenafil citrate with Doxazosin
In the third study, a single oral dose of sildenafil citrate 100 mg or matching placebo was administered in a 3-period crossover design to 20 generally healthy males with BPH. In dose period 1, subjects were administered open-label doxazosin and a single dose of sildenafil citrate 50 mg simultaneously, after at least 14 consecutive days of doxazosin. If a subject did not successfully complete this first dosing period, he was discontinued from the study. Subjects who had successfully completed the previous doxazosin interaction study (using sildenafil citrate 50 mg), including no significant hemodynamic adverse events, were allowed to skip dose period 1. Treatment with doxazosin continued for at least 7 days after dose period 1. Thereafter, sildenafil citrate 100 mg or matching placebo was administered simultaneously with doxazosin 4 mg (14 subjects) or doxazosin 8 mg (6 subjects) in standard crossover fashion. The mean subject age in this study was 66.4 years.
Twenty-five subjects were screened. Two were discontinued after study period 1: one failed to meet pre-dose screening qualifications and the other experienced symptomatic hypotension as a moderately severe adverse event 30 minutes after dosing with open-label sildenafil citrate 50 mg. Of the twenty subjects who were ultimately assigned to treatment, a total of 13 subjects successfully completed dose period 1, and seven had successfully completed the previous doxazosin study (using sildenafil citrate 50 mg).
For the 20 subjects who received sildenafil citrate 100 mg and matching placebo, the placebo-subtracted mean maximum decreases from baseline (95% CI) in systolic blood pressure were as follows:
Placebo-subtracted mean maximum decrease in systolic blood pressure (mm Hg) Sildenafil citrate 100 mg Supine 7.9 (4.6, 11.1) Standing 4.3 (-1.8,10.3) The mean profiles of the change from baseline in standing systolic blood pressure in subjects treated with doxazosin in combination with 100 mg sildenafil citrate or matching placebo are shown in Figure 4.
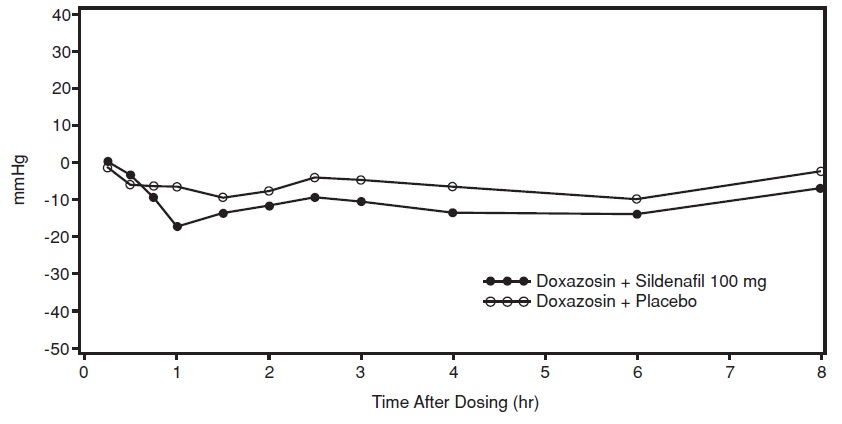
Figure 4: Mean Standing Systolic Blood Pressure Change from Baseline
Blood pressure was measured after administration of sildenafil citrate at the same times as those specified for the previous doxazosin studies. There were three subjects who had a standing SBP of < 85 mmHg. All three were taking sildenafil citrate 100 mg, and all three reported mild adverse events at the time of reductions in standing SBP, including vasodilation and lightheadedness. There were four subjects with a decrease from baseline in standing systolic BP > 30 mmHg following sildenafil citrate 100 mg, one subject with a decrease from baseline in standing systolic BP > 30 mmHg following placebo and one subject with a decrease from baseline in standing systolic BP > 30 mmHg following both sildenafil citrate and placebo. While there were no severe adverse events potentially related to blood pressure reported in this study, one subject reported moderate vasodilatation after both sildenafil citrate 50 mg and 100 mg. There were no episodes of syncope reported in this study.
Effect of Sildenafil citrate on Blood Pressure When Co-administered with Anti-hypertensives:When sildenafil citrate 100 mg oral was co-administered with amlodipine, 5 mg or 10 mg oral, to hypertensive patients, the mean additional reduction on supine blood pressure was 8 mmHg systolic and 7 mmHg diastolic.
Effect of Sildenafil citrate on Blood Pressure When Co-administered with Alcohol:Sildenafil citrate (50 mg) did not potentiate the hypotensive effect of alcohol (0.5 g/kg) in healthy volunteers with mean maximum blood alcohol levels of 0.08%. The maximum observed decrease in systolic blood pressure was -18.5 mmHg when sildenafil was co-administered with alcohol versus -17.4 mmHg when alcohol was administered alone. The maximum observed decrease in diastolic blood pressure was -17.2 mmHg when sildenafil was co-administered with alcohol versus -11.1 mmHg when alcohol was administered alone. There were no reports of postural dizziness or orthostatic hypotension. The maximum recommended dose of 100 mg sildenafil was not evaluated in this study [ see Drug Interactions (7.5)].
Effects of Sildenafil citrate on Cardiac Parameters:Single oral doses of sildenafil up to 100 mg produced no clinically relevant changes in the ECGs of normal male volunteers.
Studies have produced relevant data on the effects of sildenafil citrate on cardiac output. In one small, open-label, uncontrolled, pilot study, eight patients with stable ischemic heart disease underwent Swan-Ganz catheterization. A total dose of 40 mg sildenafil was administered by four intravenous infusions.
The results from this pilot study are shown in Table 3; the mean resting systolic and diastolic blood pressures decreased by 7% and 10% compared to baseline in these patients. Mean resting values for right atrial pressure, pulmonary artery pressure, pulmonary artery occluded pressure and cardiac output decreased by 28%, 28%, 20% and 7% respectively. Even though this total dosage produced plasma sildenafil concentrations which were approximately 2 to 5 times higher than the mean maximum plasma concentrations following a single oral dose of 100 mg in healthy male volunteers, the hemodynamic response to exercise was preserved in these patients.
Table 3. Hemodynamic Data in Patients with Stable Ischemic Heart Disease after Intravenous Administration of 40 mg of Sildenafil Means ± SD At rest After 4 minutes of exercise N Baseline (B2) n Sildenafil (D1) n Baseline n Sildenafil PAOP (mmHg) 8 8.1 ± 5.1 8 6.5 ± 4.3 8 36.0 ± 13.7 8 27.8 ± 15.3 Mean PAP (mmHg) 8 16.7 ± 4 8 12.1 ± 3.9 8 39.4 ± 12.9 8 31.7 ± 13.2 Mean RAP (mmHg) 7 5.7 ± 3.7 8 4.1 ± 3.7 - - - - Systolic SAP (mmHg) 8 150.4 ± 12.4 8 140.6 ± 16.5 8 199.5 ± 37.4 8 187.8 ± 30.0 Diastolic SAP (mmHg) 8 73.6 ± 7.8 8 65.9 ± 10 8 84.6 ± 9.7 8 79.5 ± 9.4 Cardiac output (L/min) 8 5.6 ± 0.9 8 5.2 ± 1.1 8 11.5 ± 2.4 8 10.2 ± 3.5 Heart rate (bpm) 8 67 ± 11.1 8 66.9 ± 12 8 101.9 ± 11.6 8 99.0 ± 20.4 In a double-blind study, 144 patients with erectile dysfunction and chronic stable angina limited by exercise, not receiving chronic oral nitrates, were randomized to a single dose of placebo or sildenafil citrate 100 mg 1 hour prior to exercise testing. The primary endpoint was time to limiting angina in the evaluable cohort. The mean times (adjusted for baseline) to onset of limiting angina were 423.6 and 403.7 seconds for sildenafil (N=70) and placebo, respectively. These results demonstrated that the effect of sildenafil citrate on the primary endpoint was statistically non-inferior to placebo.
Effects of Sildenafil citrate on Vision:At single oral doses of 100 mg and 200 mg, transient dose-related impairment of color discrimination was detected using the Farnsworth-Munsell 100-hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina. Subjects in the study reported this finding as difficulties in discriminating blue/green. An evaluation of visual function at doses up to twice the maximum recommended dose revealed no effects of sildenafil citrate on visual acuity, intraocular pressure, or pupillometry.
Effects of Sildenafil citrate on Sperm:There was no effect on sperm motility or morphology after single 100 mg oral doses of sildenafil citrate in healthy volunteers.
Close12.3 Pharmacokinetics
Sildenafil citrate is rapidly absorbed after oral administration, with a mean absolute bioavailability of 41% (range 25 to 63%). The pharmacokinetics of sildenafil are dose-proportional over the recommended dose range. It is eliminated predominantly by hepatic metabolism (mainly CYP3A4) and is converted to an active metabolite with properties similar to the parent, sildenafil. Both sildenafil and the metabolite have terminal half lives of about 4 hours.
Mean sildenafil plasma concentrations measured after the administration of a single oral dose of 100 mg to healthy male volunteers is depicted below:
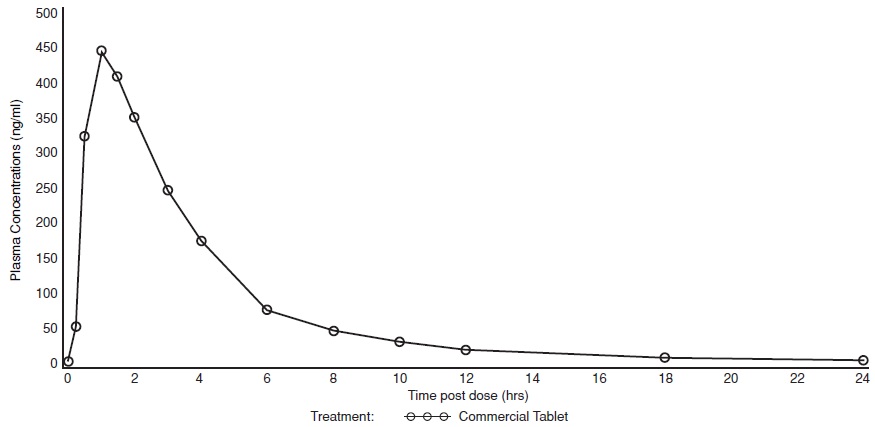
Figure 5: Mean Sildenafil Plasma Concentrations in Healthy Male Volunteers.
Absorption and Distribution:Sildenafil citrate is rapidly absorbed. Maximum observed plasma concentrations are reached within 30 to 120 minutes (median 60 minutes) of oral dosing in the fasted state. When sildenafil citrate is taken with a high fat meal, the rate of absorption is reduced, with a mean delay in T maxof 60 minutes and a mean reduction in C maxof 29%. The mean steady state volume of distribution (Vss) for sildenafil is 105 L, indicating distribution into the tissues. Sildenafil and its major circulating N-desmethyl metabolite are both approximately 96% bound to plasma proteins. Protein binding is independent of total drug concentrations.
Based upon measurements of sildenafil in semen of healthy volunteers 90 minutes after dosing, less than 0.001% of the administered dose may appear in the semen of patients.
Metabolism and Excretion:Sildenafil is cleared predominantly by the CYP3A4 (major route) and CYP2C9 (minor route) hepatic microsomal isoenzymes. The major circulating metabolite results from N-desmethylation of sildenafil, and is itself further metabolized. This metabolite has a PDE selectivity profile similar to sildenafil and an in vitropotency for PDE5 approximately 50% of the parent drug. Plasma concentrations of this metabolite are approximately 40% of those seen for sildenafil, so that the metabolite accounts for about 20% of sildenafil’s pharmacologic effects.
After either oral or intravenous administration, sildenafil is excreted as metabolites predominantly in the feces (approximately 80% of administered oral dose) and to a lesser extent in the urine (approximately 13% of the administered oral dose). Similar values for pharmacokinetic parameters were seen in normal volunteers and in the patient population, using a population pharmacokinetic approach.
Pharmacokinetics in Special Populations
Geriatrics:Healthy elderly volunteers (65 years or over) had a reduced clearance of sildenafil, resulting in approximately 84% and 107% higher plasma AUC values of sildenafil and its active N-desmethyl metabolite, respectively, compared to those seen in healthy younger volunteers (18 to 45 years). Due to age-differences in plasma protein binding, the corresponding increase in the AUC of free (unbound) sildenafil and its active N-desmethyl metabolite were 45% and 57%, respectively [ see Dosage and Administration (2.5), and Use in Specific Populations (8.5)]
Renal Impairment:In volunteers with mild (CLcr=50 to 80 mL/min) and moderate (CLcr=30 to 49 mL/min) renal impairment, the pharmacokinetics of a single oral dose of sildenafil citrate (50 mg) were not altered. In volunteers with severe (CLcr <30 mL/min) renal impairment, sildenafil clearance was reduced, resulting in approximately doubling of AUC and C maxcompared to age-matched volunteers with no renal impairment [ see Dosage and Administration (2.5), and Use in Specific Populations (8.6)].
In addition, N-desmethyl metabolite AUC and C maxvalues significantly increased by 200% and 79%, respectively in subjects with severe renal impairment compared to subjects with normal renal function.
Hepatic Impairment:In volunteers with hepatic impairment (Child-Pugh Class A and B), sildenafil clearance was reduced, resulting in increases in AUC (85%) and C max(47%) compared to age-matched volunteers with no hepatic impairment. The pharmacokinetics of sildenafil in patients with severely impaired hepatic function (Child-Pugh Class C) have not been studied [ see Dosage and Administration (2.5), and Use in Specific Populations (8.7)].
Therefore, age >65, hepatic impairment and severe renal impairment are associated with increased plasma levels of sildenafil. A starting oral dose of 25 mg should be considered in those patients [ see Dosage and Administration (2.5)].
Drug Interaction Studies
Effects of Other Drugs on Sildenafil citrate
Sildenafil metabolism is principally mediated by CYP3A4 (major route) and CYP2C9 (minor route). Therefore, inhibitors of these isoenzymes may reduce sildenafil clearance and inducers of these isoenzymes may increase sildenafil clearance. The concomitant use of erythromycin or strong CYP3A4 inhibitors (e.g., saquinavir, ketoconazole, itraconazole) as well as the nonspecific CYP inhibitor, cimetidine, is associated with increased plasma levels of sildenafil [ see Dosage and Administration (2.4)].
In vivostudies:
Cimetidine (800 mg), a nonspecific CYP inhibitor, caused a 56% increase in plasma sildenafil concentrations when co-administered with sildenafil citrate (50 mg) to healthy volunteers.
When a single 100 mg dose of sildenafil citrate was administered with erythromycin, a moderate CYP3A4 inhibitor, at steady state (500 mg bid for 5 days), there was a 160% increase in sildenafil C maxand a 182% increase in sildenafil AUC. In addition, in a study performed in healthy male volunteers, co-administration of the HIV protease inhibitor saquinavir, also a CYP3A4 inhibitor, at steady state (1200 mg tid) with sildenafil citrate (100 mg single dose) resulted in a 140% increase in sildenafil C maxand a 210% increase in sildenafil AUC. Sildenafil citrate had no effect on saquinavir pharmacokinetics. A stronger CYP3A4 inhibitor such as ketoconazole or itraconazole could be expected to have greater effect than that seen with saquinavir. Population pharmacokinetic data from patients in clinical trials also indicated a reduction in sildenafil clearance when it was co-administered with CYP3A4 inhibitors (such as ketoconazole, erythromycin, or cimetidine) [ see Dosage and Administration (2.4)and Drug Interactions (7.4)].
In another study in healthy male volunteers, co-administration with the HIV protease inhibitor ritonavir, which is a highly potent P450 inhibitor, at steady state (500 mg bid) with sildenafil citrate (100 mg single dose) resulted in a 300% (4-fold) increase in sildenafil C maxand a 1000% (11-fold) increase in sildenafil plasma AUC. At 24 hours the plasma levels of sildenafil were still approximately 200 ng/mL, compared to approximately 5 ng/mL when sildenafil was dosed alone. This is consistent with ritonavir’s marked effects on a broad range of P450 substrates. Sildenafil citrate had no effect on ritonavir pharmacokinetics [ see Dosage and Administration (2.4)and Drug Interactions (7.4)].
Although the interaction between other protease inhibitors and sildenafil has not been studied, their concomitant use is expected to increase sildenafil levels.
In a study of healthy male volunteers, co-administration of sildenafil at steady state (80 mg t.i.d.) with endothelin receptor antagonist bosentan (a moderate inducer of CYP3A4, CYP2C9 and possibly of CYP2C19) at steady state (125 mg b.i.d.) resulted in a 63% decrease of sildenafil AUC and a 55% decrease in sildenafil C max. Concomitant administration of strong CYP3A4 inducers, such as rifampin, is expected to cause greater decreases in plasma levels of sildenafil.
Single doses of antacid (magnesium hydroxide/aluminum hydroxide) did not affect the bioavailability of sildenafil citrate. In healthy male volunteers, there was no evidence of a clinically significant effect of azithromycin (500 mg daily for 3 days) on the systemic exposure of sildenafil or its major circulating metabolite.
Pharmacokinetic data from patients in clinical trials showed no effect on sildenafil pharmacokinetics of CYP2C9 inhibitors (such as tolbutamide, warfarin), CYP2D6 inhibitors (such as selective serotonin reuptake inhibitors, tricyclic antidepressants), thiazide and related diuretics, ACE inhibitors, and calcium channel blockers. The AUC of the active metabolite, N-desmethyl sildenafil, was increased 62% by loop and potassium-sparing diuretics and 102% by nonspecific beta-blockers. These effects on the metabolite are not expected to be of clinical consequence.
Effects of Sildenafil citrate on Other Drugs
In vitrostudies:
Sildenafil is a weak inhibitor of the CYP isoforms 1A2, 2C9, 2C19, 2D6, 2E1 and 3A4 (IC50 >150 µM). Given sildenafil peak plasma concentrations of approximately 1 µM after recommended doses, it is unlikely that sildenafil citrate will alter the clearance of substrates of these isoenzymes.
In vivostudies:
No significant interactions were shown with tolbutamide (250 mg) or warfarin (40 mg), both of which are metabolized by CYP2C9.
In a study of healthy male volunteers, sildenafil (100 mg) did not affect the steady state pharmacokinetics of the HIV protease inhibitors, saquinavir and ritonavir, both of which are CYP3A4 substrates.
Sildenafil citrate (50 mg) did not potentiate the increase in bleeding time caused by aspirin (150 mg).
Sildenafil at steady state, at a dose not approved for the treatment of erectile dysfunction (80 mg t.i.d.) resulted in a 50% increase in AUC and a 42% increase in C maxof bosentan (125 mg b.i.d.).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Sildenafil was not carcinogenic when administered to rats for 24 months at a dose resulting in total systemic ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Sildenafil was not carcinogenic when administered to rats for 24 months at a dose resulting in total systemic drug exposure (AUCs) for unbound sildenafil and its major metabolite of 20- and 38- times, for male and female rats, respectively, the exposures observed in human males given the Maximum Recommended Human Dose (MRHD) of 100 mg. Sildenafil was not carcinogenic when administered to mice for 18 to 21 months at dosages up to the Maximum Tolerated Dose (MTD) of 10 mg/kg/day, approximately 0.4 times the MRHD on a mg/m2 basis in a 50 kg subject.
-
14 CLINICAL STUDIES
In clinical studies, sildenafil citrate was assessed for its effect on the ability of men with erectile dysfunction (ED) to engage in sexual activity and in many cases specifically on the ability ...
In clinical studies, sildenafil citrate was assessed for its effect on the ability of men with erectile dysfunction (ED) to engage in sexual activity and in many cases specifically on the ability to achieve and maintain an erection sufficient for satisfactory sexual activity. Sildenafil citrate was evaluated primarily at doses of 25 mg, 50 mg and 100 mg in 21 randomized, double-blind, placebo-controlled trials of up to 6 months in duration, using a variety of study designs (fixed dose, titration, parallel, crossover). Sildenafil citrate was administered to more than 3,000 patients aged 19 to 87 years, with ED of various etiologies (organic, psychogenic, mixed) with a mean duration of 5 years. Sildenafil citrate demonstrated statistically significant improvement compared to placebo in all 21 studies. The studies that established benefit demonstrated improvements in success rates for sexual intercourse compared with placebo.
Efficacy Endpoints in Controlled Clinical Studies
The effectiveness of sildenafil citrate was evaluated in most studies using several assessment instruments. The primary measure in the principal studies was a sexual function questionnaire (the International Index of Erectile Function - IIEF) administered during a 4-week treatment-free run-in period, at baseline, at follow-up visits, and at the end of double-blind, placebo-controlled, at-home treatment. Two of the questions from the IIEF served as primary study endpoints; categorical responses were elicited to questions about (1) the ability to achieve erections sufficient for sexual intercourse and (2) the maintenance of erections after penetration. The patient addressed both questions at the final visit for the last 4 weeks of the study. The possible categorical responses to these questions were (0) no attempted intercourse, (1) never or almost never, (2) a few times, (3) sometimes, (4) most times, and (5) almost always or always. Also collected as part of the IIEF was information about other aspects of sexual function, including information on erectile function, orgasm, desire, satisfaction with intercourse, and overall sexual satisfaction. Sexual function data were also recorded by patients in a daily diary.
In addition, patients were asked a global efficacy question and an optional partner questionnaire was administered.
Efficacy Results from Controlled Clinical Studies
The effect on one of the major end points, maintenance of erections after penetration, is shown in Figure 6, for the pooled results of 5 fixed-dose, dose-response studies of greater than one month duration, showing response according to baseline function. Results with all doses have been pooled, but scores showed greater improvement at the 50 and 100 mg doses than at 25 mg. The pattern of responses was similar for the other principal question, the ability to achieve an erection sufficient for intercourse. The titration studies, in which most patients received 100 mg, showed similar results. Figure 6 shows that regardless of the baseline levels of function, subsequent function in patients treated with sildenafil citrate was better than that seen in patients treated with placebo. At the same time, on-treatment function was better in treated patients who were less impaired at baseline.
Effect of Sildenafil citrate on Maintenance of Erection by Baseline Score
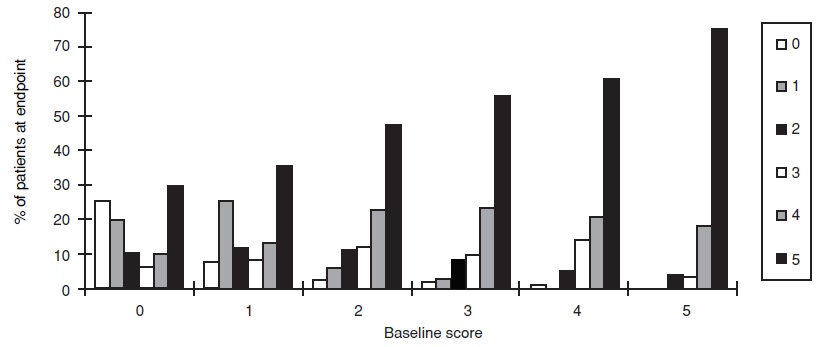
Effect of Placebo on Maintenance of Erection by Baseline Score
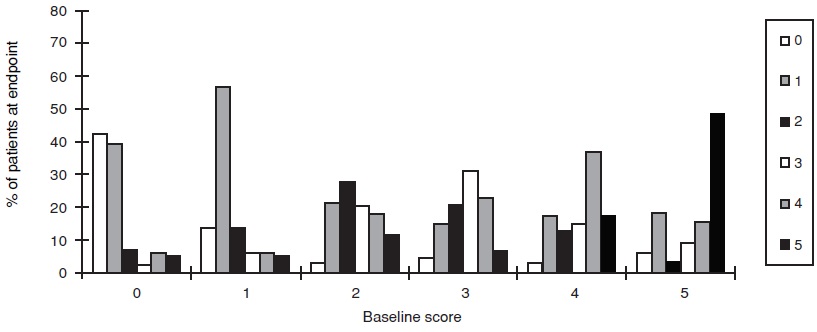
Figure 6. Effect of Sildenafil Citrate and Placebo on Maintenance of Erection by Baseline Score.
The frequency of patients reporting improvement of erections in response to a global question in four of the randomized, double-blind, parallel, placebo-controlled fixed dose studies (1,797 patients) of 12 to 24 weeks duration is shown in Figure 7. These patients had erectile dysfunction at baseline that was characterized by median categorical scores of 2 (a few times) on principal IIEF questions. Erectile dysfunction was attributed to organic (58%; generally not characterized, but including diabetes and excluding spinal cord injury), psychogenic (17%), or mixed (24%) etiologies. Sixty-three percent, 74%, and 82% of the patients on 25 mg, 50 mg and 100 mg of sildenafil citrate, respectively, reported an improvement in their erections, compared to 24% on placebo. In the titration studies (n=644) (with most patients eventually receiving 100 mg), results were similar.
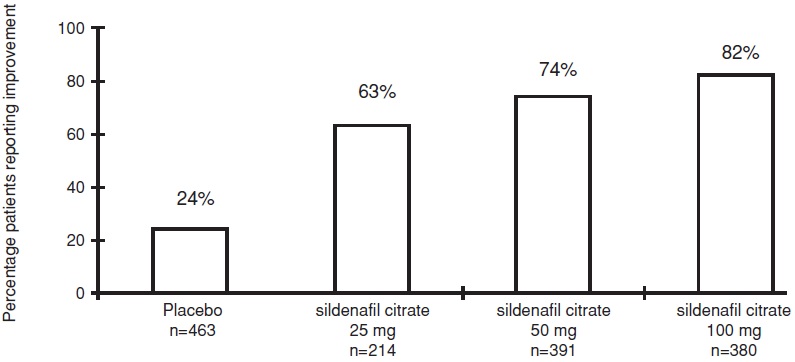
Overall treatment p<0.0001
Figure 7. Percentage of Patients Reporting an Improvement in Erections
The patients in studies had varying degrees of ED. One-third to one-half of the subjects in these studies reported successful intercourse at least once during a 4-week, treatment-free run-in period.
In many of the studies, of both fixed dose and titration designs, daily diaries were kept by patients. In these studies, involving about 1,600 patients, analyses of patient diaries showed no effect of sildenafil citrate on rates of attempted intercourse (about 2 per week), but there was clear treatment-related improvement in sexual function: per patient weekly success rates averaged 1.3 on 50 to 100 mg of sildenafil citrate vs 0.4 on placebo; similarly, group mean success rates (total successes divided by total attempts) were about 66% on sildenafil citrate vs about 20% on placebo.
During 3 to 6 months of double-blind treatment or longer-term (1 year), open-label studies, few patients withdrew from active treatment for any reason, including lack of effectiveness. At the end of the long-term study, 88% of patients reported that sildenafil citrate improved their erections.
Men with untreated ED had relatively low baseline scores for all aspects of sexual function measured (again using a 5-point scale) in the IIEF. Sildenafil citrate improved these aspects of sexual function: frequency, firmness and maintenance of erections; frequency of orgasm; frequency and level of desire; frequency, satisfaction and enjoyment of intercourse; and overall relationship satisfaction.
One randomized, double-blind, flexible-dose, placebo-controlled study included only patients with erectile dysfunction attributed to complications of diabetes mellitus (n=268). As in the other titration studies, patients were started on 50 mg and allowed to adjust the dose up to 100 mg or down to 25 mg of sildenafil citrate; all patients, however, were receiving 50 mg or 100 mg at the end of the study. There were highly statistically significant improvements on the two principal IIEF questions (frequency of successful penetration during sexual activity and maintenance of erections after penetration) on sildenafil citrate compared to placebo. On a global improvement question, 57% of sildenafil citrate patients reported improved erections versus 10% on placebo. Diary data indicated that on sildenafil citrate, 48% of intercourse attempts were successful versus 12% on placebo.
One randomized, double-blind, placebo-controlled, crossover, flexible-dose (up to 100 mg) study of patients with erectile dysfunction resulting from spinal cord injury (n=178) was conducted. The changes from baseline in scoring on the two end point questions (frequency of successful penetration during sexual activity and maintenance of erections after penetration) were highly statistically significantly in favor of sildenafil citrate. On a global improvement question, 83% of patients reported improved erections on sildenafil citrate versus 12% on placebo. Diary data indicated that on sildenafil citrate, 59% of attempts at sexual intercourse were successful compared to 13% on placebo.
Across all trials, sildenafil citrate improved the erections of 43% of radical prostatectomy patients compared to 15% on placebo.
Subgroup analyses of responses to a global improvement question in patients with psychogenic etiology in two fixed-dose studies (total n=179) and two titration studies (total n=149) showed 84% of sildenafil citrate patients reported improvement in erections compared with 26% of placebo. The changes from baseline in scoring on the two end point questions (frequency of successful penetration during sexual activity and maintenance of erections after penetration) were highly statistically significantly in favor of sildenafil citrate. Diary data in two of the studies (n=178) showed rates of successful intercourse per attempt of 70% for sildenafil citrate and 29% for placebo.
Efficacy Results in Subpopulations in Controlled Clinical Studies
A review of population subgroups demonstrated efficacy regardless of baseline severity, etiology, race and age. Sildenafil citrate was effective in a broad range of ED patients, including those with a history of coronary artery disease, hypertension, other cardiac disease, peripheral vascular disease, diabetes mellitus, depression, coronary artery bypass graft (CABG), radical prostatectomy, transurethral resection of the prostate (TURP) and spinal cord injury, and in patients taking antidepressants/antipsychotics and anti-hypertensives/diuretics.
Close -
16 HOW SUPPLIED/STORAGE AND HANDLING
Sildenafil 50 mg tablets USP are blue, round biconvex, film coated tablets, debossed with '87'on one side and plain on other side. Bottles of 15 NDC ...
Sildenafil 50 mg tablets USP are blue, round biconvex, film coated tablets, debossed with '87'on one side and plain on other side.
Bottles of 15 NDC 43063-941-15
Recommended Storage:Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Close -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information). Nitrates - Physicians should discuss with patients the contraindication of sildenafil citrate with regular ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Nitrates
Physicians should discuss with patients the contraindication of sildenafil citrate with regular and/or intermittent use of nitric oxide donors, such as organic nitrates or organic nitrites in any form [ see Contraindications (4.1)].
Guanylate Cyclase (GC) Stimulators
Physicians should discuss with patients the contraindication of sildenafil citrate with use of guanylate cyclase stimulators such as riociguat [ see Contraindications (4.3)].
Concomitant Use with Drugs Which Lower Blood Pressure
Physicians should advise patients of the potential for sildenafil citrate to augment the blood pressure lowering effect of alpha-blockers and anti-hypertensive medications. Concomitant administration of sildenafil citrate and an alpha-blocker may lead to symptomatic hypotension in some patients. Therefore, when sildenafil citrate is co-administered with alpha-blockers, patients should be stable on alpha-blocker therapy prior to initiating sildenafil citrate treatment and sildenafil citrate should be initiated at the lowest dose [ see Warnings and Precautions (5.5)].
Cardiovascular Considerations
Physicians should discuss with patients the potential cardiac risk of sexual activity in patients with preexisting cardiovascular risk factors. Patients who experience symptoms (e.g., angina pectoris, dizziness, nausea) upon initiation of sexual activity should be advised to refrain from further activity and should discuss the episode with their physician [ see Warnings and Precautions (5.1)].
Sudden Loss of Vision
Physicians should advise patients to stop use of all PDE5 inhibitors, including sildenafil citrate, and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including possible permanent loss of vision, that has been reported rarely post-marketing in temporal association with the use of all PDE5 inhibitors. Physicians should discuss with patients the increased risk of NAION in individuals who have already experienced NAION in one eye. Physicians should also discuss with patients the increased risk of NAION among the general population in patients with a “crowded” optic disc, although evidence is insufficient to support screening of prospective users of PDE5 inhibitor, including sildenafil citrate, for this uncommon condition [ see Warnings and Precautions (5.3)and Adverse Reactions (6.2)].
Sudden Hearing Loss
Physicians should advise patients to stop taking PDE5 inhibitors, including sildenafil citrate, and seek prompt medical attention in the event of sudden decrease or loss of hearing. These events, which may be accompanied by tinnitus and dizziness, have been reported in temporal association to the intake of PDE5 inhibitors, including sildenafil citrate. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [ see Warnings and Precautions (5.4)and Adverse Reactions (6.2)].
Priapism
Physicians should warn patients that prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been reported infrequently since market approval of sildenafil citrate. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result [ see Warnings and Precautions (5.2)].
Avoid Use with other PDE5 Inhibitors
Physicians should inform patients not to take sildenafil tablets, 25 mg, 50 mg and 100 mg with other PDE5 inhibitors including sildenafil tablets, 20 mg or other pulmonary arterial hypertension (PAH) treatments containing sildenafil. Sildenafil is also marketed as sildenafil tablets, 20 mg for the treatment of PAH. The safety and efficacy of sildenafil tablets, 25 mg, 50 mg and 100 mg with other PDE5 inhibitors, including sildenafil tablets, 20 mg, have not been studied [ see Warnings and Precautions (5.7)].
Sexually Transmitted Disease
The use of sildenafil citrate offers no protection against sexually transmitted diseases. Counseling of patients about the protective measures necessary to guard against sexually transmitted diseases, including the Human Immunodeficiency Virus (HIV), may be considered [ see Warnings and Precautions (5.9)].
Dispense with Patient Information available at: https://torrentpharma.com/pi/usa/products/
Manufactured by:
Torrent Pharmaceuticals LTD., India.
Manufactured for:
Torrent Pharma INC., Basking Ridge, NJ 07920.
8098159 Revised: December 2024
Close -
PATIENT INFORMATION
Dispense with Patient Information available at: https://torrentpharma.com/pi/usa/products/ Sildenafil (sil DEN a fil) Citrate Tablets USP - What is the most important information I should ...
Dispense with Patient Information available at: https://torrentpharma.com/pi/usa/products/
Sildenafil (sil DEN a fil) Citrate Tablets USP
What is the most important information I should know about sildenafil tablets?
Sildenafil tablets can cause your blood pressure to drop suddenly to an unsafe level if it is taken with certain other medicines.Do not take sildenafil tablets if you take any other medicines called “nitrates.” Nitrates are used to treat chest pain (angina). A sudden drop in blood pressure can cause you to feel dizzy, faint, or have a heart attack or stroke. Do not take sildenafil tablets if you take medicines called guanylate cyclase stimulators which include:
- Riociguat (Adempas ®) a medicine that treats pulmonary arterial hypertension and chronic-thromboembolic pulmonary hypertension.
Tell all your healthcare providers that you take Sildenafil tablets.If you need emergency medical care for a heart problem, it will be important for your healthcare provider to know when you last took Sildenafil tablets.
Stop sexual activity and get medical help right away if you get symptoms such as chest pain, dizziness, or nausea during sex.
Sexual activity can put an extra strain on your heart, especially if your heart is already weak from a heart attack or heart disease. Ask your doctor if your heart is healthy enough to handle the extra strain of having sex.
Sildenafil tablets does not protect you or your partner from getting sexually transmitted diseases, including HIV - the virus that causes AIDS.
What are sildenafil tablets?
Sildenafil tablets is a prescription medicine used to treat erectile dysfunction (ED). You will not get an erection just by taking this medicine. Sildenafil tablets helps a man with erectile dysfunction get and keep an erection only when he is sexually excited (stimulated).
Sildenafil tablets is not for use in women or children.
It is not known if Sildenafil tablets is safe and effective in women or children under 18 years of age.
Who should not take sildenafil tablet?
Do not take sildenafil tablets if you:
- take medicines called nitrates (such as nitroglycerin)
- use street drugs called “poppers” such as amyl nitrate or amyl nitrite, and butyl nitrate
- take any medicines called guanylate cyclase stimulators such as riociguat (Adempas)
- are allergic to sildenafil, as contained in Sildenafil tablets, 25 mg, 50 mg and 100 mg and Sildenafil tablets, 20 mg or any of the ingredients in Sildenafil tablets. See the end of this leaflet for a complete list of ingredients in Sildenafil tablets.
What should I tell my healthcare provider before taking sildenafil tablets?
Before you take Sildenafil tablets, tell your healthcare provider if you:
- have or have had heart problems such as a heart attack, irregular heartbeat, angina, chest pain, narrowing of the aortic valve or heart failure
- have had heart surgery within the last 6 months
- have pulmonary hypertension
- have had a stroke
- have low blood pressure, or high blood pressure that is not controlled
- have a deformed penis shape
- have had an erection that lasted for more than 4 hours
- have problems with your blood cells such as sickle cell anemia, multiple myeloma, or leukemia
- have retinitis pigmentosa, a rare genetic (runs in families) eye disease
- have ever had severe vision loss, including an eye problem called non-arteritic anterior ischemic optic neuropathy (NAION)
- have bleeding problems
- have or have had stomach ulcers
- have liver problems
- have kidney problems or are having kidney dialysis
- have any other medical conditions
Tell your healthcare provider about all the medicines you take*,including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Sildenafil tablets may affect the way other medicines work, and other medicines may affect the way Sildenafil tablets works causing side effects. Especially tell your healthcare provider if you take any of the following:
- medicines called nitrates (see “What is the most important information I should know about Sildenafil tablets?”)
- medicines called guanylate cyclase stimulators, such as riociguat (Adempas)
- medicines called alpha blockers such as Hytrin (terazosin HCl), Flomax (tamsulosin HCl), Cardura (doxazosin mesylate), Minipress (prazosin HCl), Uroxatral (alfuzosin HCl), Jalyn (dutasteride and tamsulosin HCl), or Rapaflo (silodosin). Alpha-blockers are sometimes prescribed for prostate problems or high blood pressure. In some patients, the use of sildenafil tablets with alpha-blockers can lead to a drop in blood pressure or to fainting.
- medicines called HIV protease inhibitors, such as ritonavir (Norvir), indinavir sulfate (Crixivan), saquinavir (Fortovase or Invirase) or atazanavir sulfate (Reyataz)
- some types of oral antifungal medicines, such as ketoconazole (Nizoral), and itraconazole (Sporanox)
- some types of antibiotics, such as clarithromycin (Biaxin), telithromycin (Ketek), or erythromycin
- other medicines that treat high blood pressure
- other medicines or treatments for ED
- Sildenafil tablets, 25 mg, 50 mg and 100 mg contains sildenafil, which is the same medicine found in another drug called Sildenafil tablets, 20 mg. Sildenafil tablets, 20 mg is used to treat a rare disease called pulmonary arterial hypertension (PAH). Sildenafil tablets, 25 mg, 50 mg and 100 mg should not be used with Sildenafil tablets, 20 mg or with other PAH treatments containing sildenafil or any other PDE5 inhibitors (such as Adcirca [tadalafil]).
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take sildenafil tablets?
- Take sildenafil tablets exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much sildenafil tablets to take and when to take it.
- Your healthcare provider may change your dose if needed.
- Take sildenafil tablets about 1 hour before sexual activity. You may take sildenafil tablets between 30 minutes to 4 hours before sexual activity if needed.
- Sildenafil tablets can be taken with or without food. If you take sildenafil tablets after a high fat meal (such as a cheeseburger and french fries), sildenafil tablets may take a little longer to start working
- Do nottake sildenafil tablets more than 1 time a day.
- If you accidentally take too much sildenafil tablets, call your doctor or go to the nearest hospital emergency room right away.
What are the possible side effects of sildenafil tablets?
Sildenafil tablets can cause serious side effects.Rarely reported side effects include:
- an erection that will not go away (priapism).If you have an erection that lasts more than 4 hours, get medical help right away. If it is not treated right away, priapism can permanently damage your penis.
- sudden vision loss in one or both eyes.Sudden vision loss in one or both eyes can be a sign of a serious eye problem called non-arteritic anterior ischemic optic neuropathy (NAION). It is uncertain whether PDE5 inhibitors directly cause the vision loss. Stop taking sildenafil tablets and call your healthcare provider right away if you have sudden vision loss in one or both eyes.
- sudden hearing decrease or hearing loss.Some people may also have ringing in their ears (tinnitus) or dizziness. If you have these symptoms, stop taking sildenafil tablets and contact a doctor right away.
The most common side effects of sildenafil tablets are:
- headache
- flushing
- upset stomach
- abnormal vision, such as changes in color vision (such as having a blue color tinge) and blurred vision
- stuffy or runny nose
- back pain
- muscle pain
- nausea
- dizziness
- rash
In addition, heart attack, stroke, irregular heartbeats and death have happened rarely in men taking sildenafil tablets. Most, but not all, of these men had heart problems before taking sildenafil tablets. It is not known if sildenafil tablets caused these problems.
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of sildenafil tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store sildenafil tablets?
- Sildenafil Tablets comes in child-resistant package.
- Store sildenafil tablets at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Keep sildenafil tablets and all medicines out of the reach of children.
General information about the safe and effective use of sildenafil tablets
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use sildenafil tablets for a condition for which it was not prescribed. Do not give sildenafil tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about sildenafil tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about sildenafil tablets that is written for health professionals.
For more information, Call 1-800-912-9561.
What are the ingredients in sildenafil tablets?
Active ingredients:sildenafil citrate, USP
Inactive ingredients:croscarmellose sodium, dibasic calcium phosphate anhydrous, hypromellose, lake of indigo carmine, microcrystalline cellulose, sodium stearyl fumarate, titanium dioxide and triacetin.
This product’s label may have been updated. For current full prescribing information, please visit www.torrentpharma.com
Trademarks are the property of their respective owners.
This Patient Information has been approved by the U.S. Food and Drug Administration
Manufactured by:
Torrent Pharmaceuticals LTD., India.
Manufactured for:
Torrent Pharma INC., Basking Ridge, NJ 07920.
8098160 Revised: December 2024
Close -
PACKAGE LABEL/PRINCIPAL DISPLAY PANEL - 50 mgSildenafil Tablets, USP 50 mg
-
INGREDIENTS AND APPEARANCEProduct Information
SILDENAFIL CITRATE sildenafil tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43063-941(NDC:13668-187) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILDENAFIL CITRATE (UNII: BW9B0ZE037) (SILDENAFIL - UNII:3M7OB98Y7H) SILDENAFIL 50 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color blue Score no score Shape ROUND (Biconvex) Size 10mm Flavor Imprint Code 87 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43063-941-15 15 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091448 06/11/2018 Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) Registrant - PD-Rx Pharmaceuticals, Inc. (156893695)
CloseEstablishment Name Address ID/FEI Business Operations PD-Rx Pharmaceuticals, Inc. 156893695 repack(43063-941)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
SILDENAFIL CITRATE- sildenafil tablet, film coated
Number of versions: 11
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Apr 17, 2025 | 23 (current) | download |
| Jan 6, 2025 | 22 | download |
| Oct 18, 2024 | 21 | download |
| Apr 26, 2023 | 20 | download |
| Aug 31, 2022 | 19 | download |
| Jul 15, 2022 | 18 | download |
| Apr 14, 2022 | 17 | download |
| Dec 13, 2021 | 16 | download |
| May 6, 2020 | 15 | download |
| May 3, 2019 | 14 | download |
| Jan 31, 2019 | 4 | download |
Get Label RSS Feed for this Drug
SILDENAFIL CITRATE- sildenafil tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=78c4551c-b922-43c9-9a5a-66377ea07bc2
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
SILDENAFIL CITRATE- sildenafil tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 43063-941-15 |