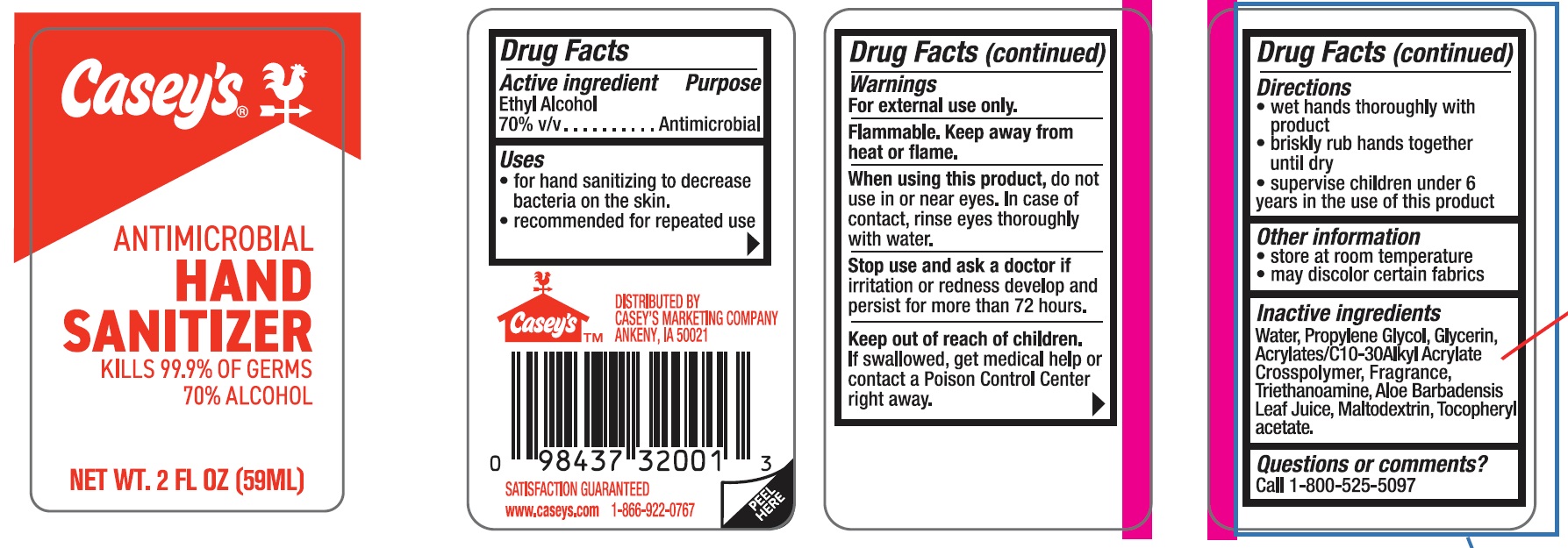
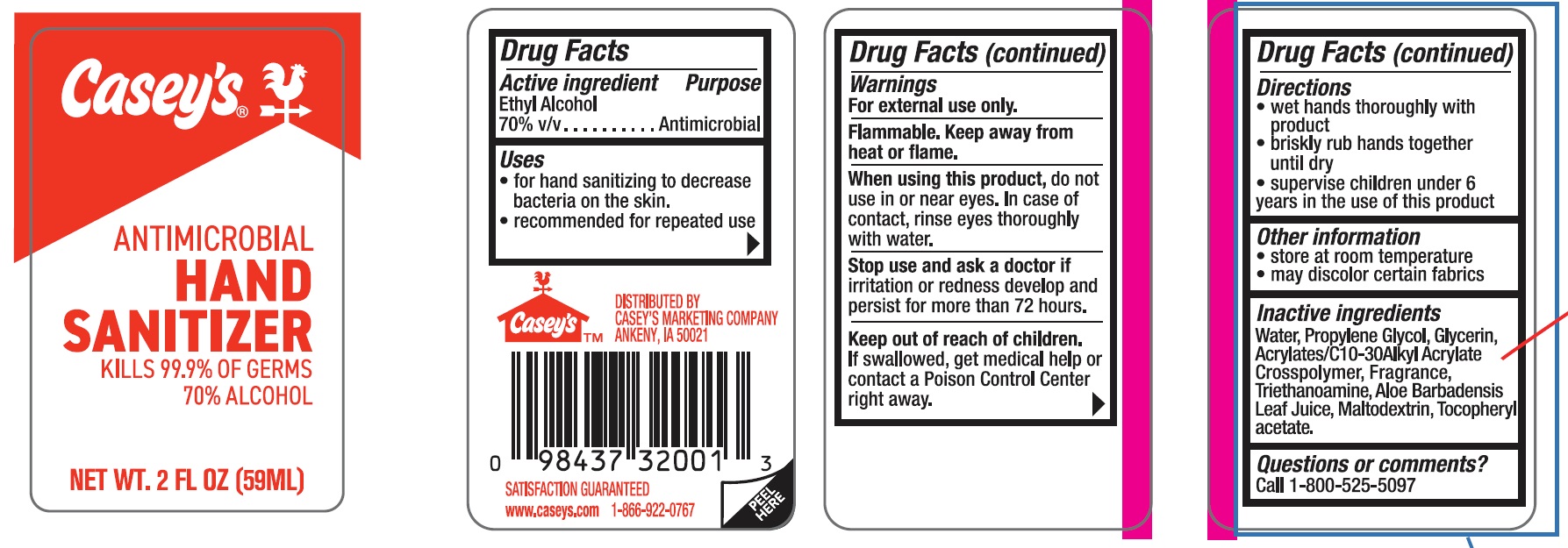
Label: CASEYS ANTIMICROBIAL HAND SANITIZER- ethyl alcohol gel
- NDC Code(s): 67751-071-01, 67751-071-02
- Packager: Navajo Manufacturing Company Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CASEYS ANTIMICROBIAL HAND SANITIZER
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67751-071 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) TOCOPHERYL GLUCOSIDE (UNII: 9CKD1JE38R) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALOE ARBORESCENS LEAF (UNII: 09TD8L5SQV) TRICLOSAN (UNII: 4NM5039Y5X) Product Characteristics Color Score Shape Size Flavor APPLE (candy apple) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67751-071-01 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/29/2024 2 NDC:67751-071-02 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 04/29/2024 Labeler - Navajo Manufacturing Company Inc. (091917799) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 manufacture(67751-071)