Label: 0.9% SODIUM CHLORIDE irrigant
- NDC Code(s): 51662-1301-1
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 0409-7138
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 21, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED
-
DESCRIPTION
These products are sterile, nonpyrogenic solutions of electrolytes in water for injection intended only for sterile irrigation, washing, rinsing and dilution purposes.
Each 100 mL of 0.45% Sodium Chloride Irrigation, USP contains: Sodium chloride 450 mg; pH 5.6 (4.5 to 7.0). The solution is hypotonic (154 mOsmol/liter, CALC.) and has the following electrolyte content (mEq/liter): Na+ 77; Cl− 77.
Each 100 mL of 0.9% Sodium Chloride Irrigation, USP contains: Sodium chloride 900 mg; pH 5.6 (4.5 to 7.0). May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. The solution is isotonic (308 mOsmol/liter, CALC.) and has the following electrolyte content (mEq/liter): Na+ 154; Cl− 154.
These irrigations contain no bacteriostat, antimicrobial agent or added buffer and are intended only for use as single-dose or short procedure irrigation. When smaller volumes are required the unused portion should be discarded.
Each of these irrigations may be classified as a sterile irrigant, wash, rinse, diluent and pharmaceutical vehicle.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly.
The semi-rigid container is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The container requires no vapor barrier to maintain the proper drug concentration.
Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
-
CLINICAL PHARMACOLOGY
Each of these irrigation solutions exert a mechanical cleansing action for sterile irrigation of body cavities, tissues or wounds, indwelling urethral catheters and surgical drainage tubes and for washing, rinsing or soaking surgical dressings, instruments and laboratory specimens. Each also serves as a diluent or vehicle for drugs used for irrigation or other pharmaceutical preparations.
0.45% Sodium Chloride Irrigation, USP provides a hypotonic half-strength saline irrigation identical in composition with 0.45% Sodium Chloride Injection, USP.
0.9% Sodium Chloride Irrigation, USP provides an isotonic saline irrigation identical in composition with 0.9% Sodium Chloride Injection, USP (normal saline).
0.9% Sodium Chloride Irrigation, USP is considered generally compatible with living tissues and organs; 0.45% Sodium Chloride Irrigation, USP may be used alone or combined with appropriate additives when 0.9% sodium chloride is considered too irritating for wounds or other altered structures.
Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl−) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Chloride (Cl−) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells. The distribution and excretion of sodium (Na+) and chloride (Cl−) are largely under the control of the kidney which maintains a balance between intake and output.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
WARNINGS
FOR IRRIGATION ONLY. NOT FOR INJECTION.
Entry of a hypotonic solution into the circulation may cause hemolysis.
Irrigating fluids have been demonstrated to enter the systemic circulation in relatively large volumes; thus each of these irrigations must be regarded as a systemic drug. Absorption of large amounts can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentrations of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
Do not heat container over 66°C (150°F).
-
PRECAUTIONS
Do not use for irrigation that may result in absorption into the blood.
Caution should be observed when a hypotonic solution is used for continuous irrigation or allowed to "dwell" inside body cavities because of possible absorption into the blood stream and the production of intravascular hemolysis and circulatory overload.
Aseptic technique is essential with the use of sterile solutions for irrigation of body cavities, wounds and urethral catheters or for wetting dressings that come in contact with body tissues.
When used as a "pour" irrigation, no part of the contents should be allowed to contact the surface below the outer protected thread area of the semi-rigid wide mouth container. The flexible container is designed for use with nonvented irrigation sets. When used for irrigation via irrigation equipment, the administration set should be attached promptly. Unused portions should be discarded and a fresh container of appropriate size used for the start-up of each cycle or repeat procedure. For repeated irrigations of urethral catheters, a separate container should be used for each patient.
Do not administer unless solution is clear, seal is intact and container is undamaged.
Discard unused portion.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies with Sodium Chloride Irrigation, USP have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Nursing Mothers:
Caution should be exercised when Sodium Chloride Irrigation, USP is administered to a nursing woman.
Pregnancy:
Teratogenic Effects.
Pregnancy Category C. Animal reproduction studies have not been conducted with Sodium Chloride Irrigation, USP. It is also not known whether Sodium Chloride Irrigation, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Chloride Irrigation, USP should be given to a pregnant woman only if clearly needed.
Pediatric Use:
Safety and effectiveness of Sodium Chloride irrigation solution in pediatric patients have not been established by adequate and well-controlled trials. However, the use of Sodium Chloride irrigation solution in the pediatric population is referenced in the medical literature. The Warnings, Precautions, and Adverse Reactions identified in the label should be observed in the pediatric population.
Geriatric Use:
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients.
This drug is known to be substantially secreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Possible adverse effects arising from the irrigation of body cavities, tissues, or indwelling catheters and tubes are usually avoidable when proper procedures are followed. Displaced catheters or drainage tubes can lead to irrigation or infiltration of unintended structures or cavities. Excessive volume or pressure during irrigation of closed cavities may cause undue distension or disruption of tissues. Accidental contamination from careless technique may transmit infection.
Should any adverse reaction occur, discontinue the irrigant, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
In the event of overhydration or solute overload, re-evaluate the patient and institute appropriate corrective measures. See WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.
-
DOSAGE & ADMINISTRATION
The dose is dependent upon the capacity or surface area of the structure to be irrigated and the nature of the procedure. When used as a diluent or vehicle for other drugs, the manufacturer's recommendations should be followed.
Drug Interactions
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution container permits. See PRECAUTIONS.
-
HOW SUPPLIED
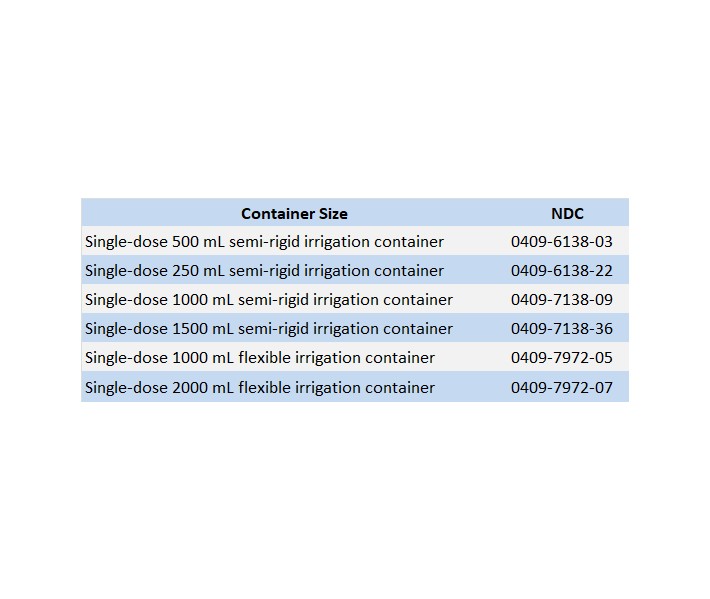
0.9% SODIUM CHLORIDE IRRIGATION, USP is supplied in the following dosage forms.
NDC 51662-1301-1
0.9% SODIUM CHLORIDE IRRIGATION, USP 1000mL BOTTLEHF Acquisition Co LLC, DBA HealthFirst
Mukilteo, WA 98275Also supplied in the following manufacture supplied dosage forms
0.45% Sodium Chloride Irrigation, USP:

0.9% Sodium Chloride Irrigation, USP:
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Revised: May, 2009

- PRINCIPAL DISPLAY PANEL, 1000 mL BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL, SERIALIZED 1000 mL BOTTLE LABEL
-
INGREDIENTS AND APPEARANCE
0.9% SODIUM CHLORIDE
0.9% sodium chloride irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51662-1301(NDC:0409-7138) Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 900 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1301-1 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/18/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017514 10/18/2018 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 relabel(51662-1301)




