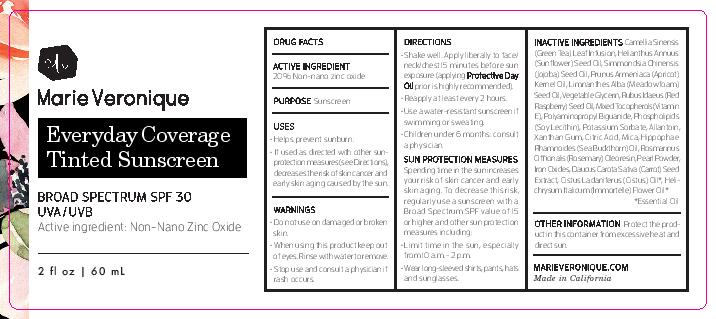
Label: EVERYDAY COVERAGE TINTED SUNSCREEN - LIGHT TINT- zinc oxide cream
- NDC Code(s): 72592-002-60
- Packager: MV Advanced
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
INACTIVE INGREDIENT
Camellia Sinensis (Green Tea) Leaf Infusion, Helianthus Annuus (Sunflower) Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Prunus Armeniaca (Apricot) Kernel Oil, Limnanthes Alba (Meadowfoam) Seed Oil, Vegetable Glycerin, Rubus Idaeus (Red Raspberry) Seed Oil, Mixed Tocopherols (Vitamin E), Polyaminopropyl Biguanide, Phospholipids (Soy Lecithin), Potassium Sorbate, Allantoin, Xanthan Gum, Citric Acid, Mica, Hippophae Rhamnoides (Sea Buckthorn) Oil, Rosmarinus Officinalis (Rosemary) Oleoresin, Pearl Powder, Iron Oxides, Daucus Carota Sativa (Carrot) Seed Extract, Cistus Ladaniferus (Cistus) Oil*, Helichrysum Italicum (Immortelle) Flower Oil*
*Essential Oil
- WARNINGS
-
DOSAGE & ADMINISTRATION
- Shake well. Apply liberally to face/neck/chest 15 minutes before sun exposure (applying Protective Day Oilprior is highly recommended).
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Children under 6 months: consult a physician.
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- STORAGE AND HANDLING
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EVERYDAY COVERAGE TINTED SUNSCREEN - LIGHT TINT
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72592-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) XANTHAN GUM (UNII: TTV12P4NEE) ROSEMARY OIL (UNII: 8LGU7VM393) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BIGUANIDE (UNII: FB4Q52I9K2) PEARL (HYRIOPSIS CUMINGII) (UNII: A75L5FZ40U) FERROUS OXIDE (UNII: G7036X8B5H) DAUCUS CAROTA SUBSP. SATIVUS SEED (UNII: 9M6AAX381U) HELICHRYSUM ITALICUM FLOWER OIL (UNII: O97ZV7726K) GREEN TEA LEAF (UNII: W2ZU1RY8B0) APRICOT KERNEL OIL (UNII: 54JB35T06A) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) SUNFLOWER OIL (UNII: 3W1JG795YI) GLYCERIN (UNII: PDC6A3C0OX) RASPBERRY SEED OIL (UNII: 9S8867952A) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HIPPOPHAE RHAMNOIDES SEED OIL (UNII: T53SBG6741) LABDANUM OIL (UNII: 67GS9BGA2X) JOJOBA OIL (UNII: 724GKU717M) MICA (UNII: V8A1AW0880) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72592-002-60 60 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 09/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2010 Labeler - MV Advanced (013130515) Establishment Name Address ID/FEI Business Operations Allure Labs, Inc. 926831603 manufacture(72592-002)