Label: DOXYCYCLINE injection, powder, lyophilized, for solution
- NDC Code(s): 71288-030-20, 71288-030-21
- Packager: Meitheal Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONmeitheal® Rx only
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of Doxycycline for Injection and other antibacterial drugs, Doxycycline for Injection should be used only to ...
-
DESCRIPTION
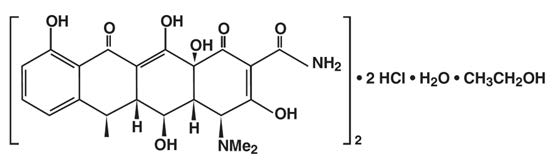
Doxycycline for Injection, USP is a sterile, lyophilized powder prepared from a solution of doxycycline hyclate, ascorbic acid and mannitol in Water for Injection. Doxycycline hyclate is a broad ...
-
CLINICAL PHARMACOLOGY
Tetracyclines are readily absorbed and are bound to plasma proteins in varying degree. They are concentrated by the liver in the bile, and excreted in the urine and feces at high concentrations ...
-
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Doxycycline for Injection and other antibacterial drugs, Doxycycline for Injection should be used only to ...
-
CONTRAINDICATIONS
This drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
WARNINGS
The use of drugs of the tetracycline class during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth ...
-
PRECAUTIONS
General - As with other antibacterial drugs, use of doxycycline may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, doxycycline should be discontinued ...
-
ADVERSE REACTIONS
Gastrointestinal - Anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis and inflammatory lesions (with monilial overgrowth) in the anogenital region, and pancreatitis ...
-
DOSAGE AND ADMINISTRATION
NOTE:Rapid administration is to be avoided. Parenteral therapy is indicated only when oral therapy is not indicated. Oral therapy should be instituted as soon as possible. If intravenous ...
-
PREPARATION OF SOLUTIONTo prepare a solution containing 10 mg per mL, the contents of the vial should be reconstituted with 10 mL (for the 100 mg per vial container) of Sterile Water for Injection or any of the 10 ...
-
HOW SUPPLIED
Doxycycline for Injection, USP is a sterile powder and is supplied as follows: NDCDoxycycline for Injection, USPPackage Factor - 71288-030-21Doxycycline hyclate equivalent to 100 mg ...
-
SPL UNCLASSIFIED SECTIONBrands listed are the trademarks of their respective owners. meitheal® Mfd. for Meitheal Pharmaceuticals - Chicago, IL 60631 (USA) ©2025 Meitheal Pharmaceuticals Inc. Mfd. by Kindos Pharmaceuticals ...
-
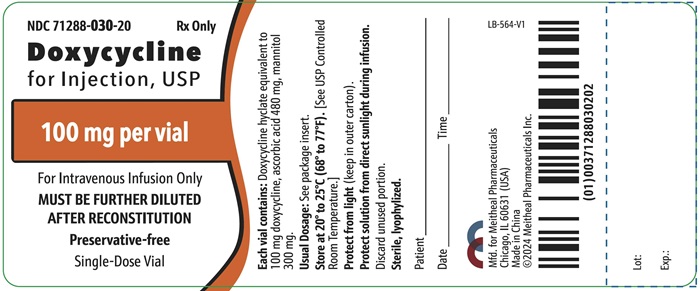
PRINCIPAL DISPLAY PANEL – Doxycycline for Injection, USP 100 mg Vial LabelNDC 71288-030-20 - Rx Only - Doxycycline for Injection, USP - 100 mg per vial - For Intravenous Infusion Only - MUST BE FURTHER DILUTED AFTER RECONSTITUTION - Preservative-free - Single-Dose ...
-
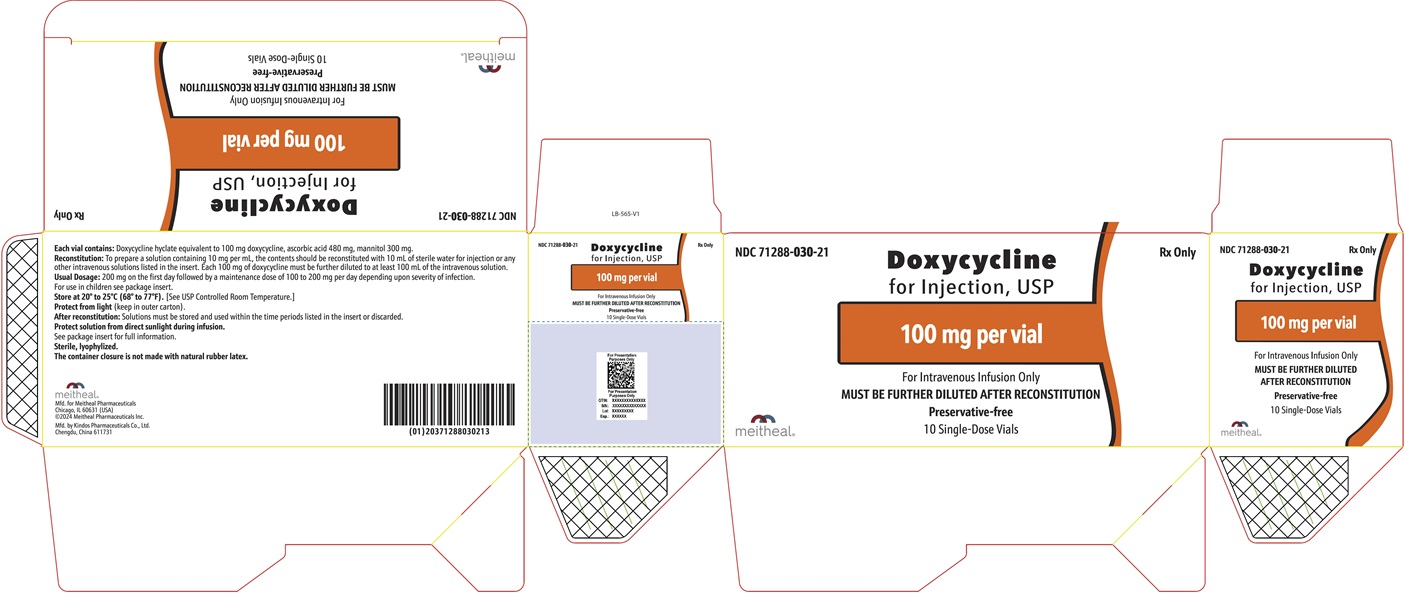
PRINCIPAL DISPLAY PANEL – Doxycycline for Injection, USP 100 mg CartonNDC 71288-030-21 - Rx Only - Doxycycline for Injection, USP - 100 mg per vial - For Intravenous Infusion Only - MUST BE FURTHER DILUTED AFTER RECONSTITUTION - Preservative-free - 10 Single-Dose ...
-
INGREDIENTS AND APPEARANCEProduct Information