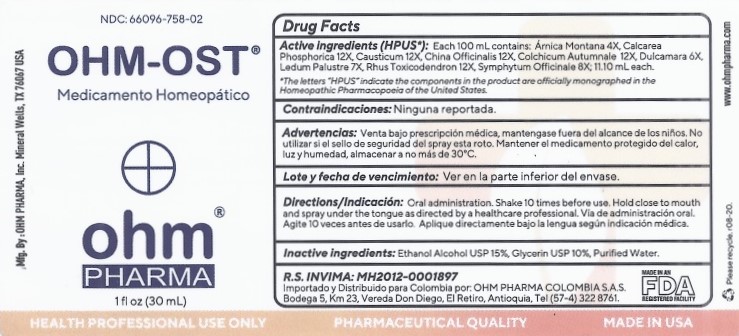
Label: OHM-OST- arnica montana, calcarea phosphorica, causticum, china officinalis, colchicum autumnale, dulcamara, ledum palustre, rhus toxicodendron, symphytum officinale spray
- NDC Code(s): 66096-758-02
- Packager: OHM PHARMA INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 25, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients (HPUS*): 100 mL contains: Arnica Montana 4X, Calcarea Phosphorica 12X, Causticum 12X, China Officinalis 12X,
Colchicum Autumnale 12X, Dulcamara 6X, Ledum Palustre 7X, Rhus Toxicodendron 12X, Symphytum Officianale 8X; 11.10 mL each.
*The letters "HPUS" indicate the components in the product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OHM-OST
arnica montana, calcarea phosphorica, causticum, china officinalis, colchicum autumnale, dulcamara, ledum palustre, rhus toxicodendron, symphytum officinale sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66096-758 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 4 [hp_X] in 30 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] in 30 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 12 [hp_X] in 30 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 12 [hp_X] in 30 mL COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 12 [hp_X] in 30 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 6 [hp_X] in 30 mL LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 7 [hp_X] in 30 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 12 [hp_X] in 30 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 8 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66096-758-02 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 12/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/19/2018 Labeler - OHM PHARMA INC. (030572478) Registrant - OHM PHARMA INC. (030572478) Establishment Name Address ID/FEI Business Operations OHM PHARMA INC. 030572478 manufacture(66096-758)