Label: ACITRETIN capsule
- NDC Code(s): 46708-741-30, 46708-742-30, 46708-743-30
- Packager: Alembic Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION

-
BOXED WARNING
(What is this?)
CONTRAINDICATIONS AND WARNINGS: Pregnancy
Acitretin must not be used by females who are pregnant, or who intend to become pregnant during therapy or at any time for at least 3 years following discontinuation of therapy. Acitretin also must not be used by females who may not use reliable contraception while undergoing treatment and for at least 3 years following discontinuation of treatment. Acitretin is a metabolite of etretinate (TEGISON), and major human fetal abnormalities have been reported with the administration of acitretin and etretinate. Potentially, any fetus exposed can be affected.
Clinical evidence has shown that concurrent ingestion of acitretin and ethanol has been associated with the formation of etretinate, which has a significantly longer elimination half-life than acitretin. Because the longer elimination half-life of etretinate would increase the duration of teratogenic potential for female patients, ethanol must not be ingested by female patients of childbearing potential either during treatment with acitretin or for 2 months after cessation of therapy. This allows for elimination of acitretin, thus removing the substrate for transesterification to etretinate. The mechanism of the metabolic process for conversion of acitretin to etretinate has not been fully defined. It is not known whether substances other than ethanol are associated with transesterification.
Acitretin has been shown to be embryotoxic and/or teratogenic in rabbits, mice, and rats at oral doses of 0.6, 3, and 15 mg per kg, respectively. These doses are approximately 0.2, 0.3, and 3 times the maximum recommended therapeutic dose, respectively, based on a mg-per-m2 comparison.
Major human fetal abnormalities associated with acitretin and/or etretinate administration have been reported including meningomyelocele; meningoencephalocele; multiple synostoses; facial dysmorphia; syndactyly; absence of terminal phalanges; malformations of hip, ankle, and forearm; low-set ears; high palate; decreased cranial volume; cardiovascular malformation; and alterations of the skull and cervical vertebrae.
Acitretin should be prescribed only by those who have special competence in the diagnosis and treatment of severe psoriasis, are experienced in the use of systemic retinoids, and understand the risk of teratogenicity.
Because of the teratogenicity of acitretin, a program called the H.A.E.R.T. program, Healthier Acitretin Education and Remission Treatment, has been developed to educate women of childbearing potential and their healthcare providers about the serious risks associated with acitretin and to help prevent pregnancies from occurring with the use of this drug and for 3 years after its discontinuation. The H.A.E.R.T. program requirements are described below and program materials are available at https://www.alembicusa.com/AcitretinHAERT or may be requested by calling 1-866-210-9797 (see also PRECAUTIONS section).
Important Information for Women of Childbearing Potential:
Acitretin should be considered only for women with severe psoriasis unresponsive to other therapies or whose clinical condition contraindicates the use of other treatments.Females of reproductive potential must not be given a prescription for acitretin until pregnancy is excluded. Acitretin are contraindicated in females of reproductive potential unless the patient meets ALL of the following conditions:
• Must have had 2 negative urine or serum pregnancy tests with a sensitivity of at least 25 mIU per mL before receiving the initial prescription for acitretin. The first test (a screening test) is obtained by the prescriber when the decision is made to pursue therapy with acitretin. The second pregnancy test (a confirmation test) should be done during the first 5 days of the menstrual period immediately preceding the beginning of therapy with acitretin. For patients with amenorrhea, the second test should be done at least 11 days after the last act of unprotected sexual intercourse (without using 2 effective forms of contraception [birth control] simultaneously). If the second pregnancy test is negative, initiation of treatment with acitretin should begin within 7 days of the specimen collection. Acitretin should be limited to a monthly supply.
• Must have a pregnancy test with a sensitivity of at least 25 mIU per mL repeated every month during treatment with acitretin. The patient must have a negative result from a urine or serum pregnancy test before receiving a prescription for acitretin. To encourage compliance with this recommendation, a monthly supply of the drug should be prescribed. For at least 3 years after discontinuing therapy with acitretin, a pregnancy test must be repeated every 3 months.
• Must have selected and have committed to use 2 effective forms of contraception (birth control) simultaneously, at least 1 of which must be a primary form, unless absolute abstinence is the chosen method, or the patient has undergone a hysterectomy or is clearly postmenopausal.
• Patients must use 2 effective forms of contraception (birth control) simultaneously for at least 1 month prior to initiation of therapy with acitretin, during therapy with acitretin, and for at least 3 years after discontinuing therapy with acitretin. Counseling about contraception and behaviors associated with an increased risk of pregnancy must be repeated on a monthly basis by the prescriber during therapy with acitretin and every 3 months for at least 3 years following discontinuation of acitretin.
Effective forms of contraception include both primary and secondary forms of contraception. Primary forms of contraception include: tubal ligation, partner’s vasectomy, intrauterine devices, birth control pills, and injectable/implantable/insertable/topical hormonal birth control products. Secondary forms of contraception include condoms (with or without spermicide), diaphragms and cervical caps (which must be used with a spermicide), and vaginal sponges (contains spermicide).
Any birth control method can fail. Therefore, it is critically important that women of childbearing potential use 2 effective forms of contraception (birth control) simultaneously. It has not been established if there is a pharmacokinetic interaction between acitretin and combined oral contraceptives. However, it has been established that acitretin interferes with the contraceptive effect of microdosed progestin preparations.1 Microdosed “minipill” progestin preparations are not recommended for use with acitretin. It is not known whether other progestin¬only contraceptives, such as implants and injectables, are adequate methods of contraception during acitretin therapy. Prescribers are advised to consult the package insert of any medication administered concomitantly with hormonal contraceptives, since some medications may decrease the effectiveness of these birth control products. Patients should be prospectively cautioned not to self-medicate with the herbal supplement St. John’s wort because a possible interaction has been suggested with hormonal contraceptives based on reports of breakthrough bleeding on oral contraceptives shortly after starting St. John’s wort. Pregnancies have been reported by users of combined hormonal contraceptives who also used some form of St. John’s wort (see PRECAUTIONS).
• Must have signed a Patient Agreement/Informed Consent for Female Patients that contains warnings about the risk of potential birth defects if the fetus is exposed to acitretin, about contraceptive failure, about the fact that they must not ingest beverages or products containing ethanol while taking acitretin and for 2 months after treatment with acitretin has been discontinued, and about preventing pregnancy while taking acitretin and for at least 3 years after discontinuing acitretin.
If pregnancy does occur during therapy with acitretin or at any time for at least 3 years following discontinuation of acitretin, the prescriber and patient should discuss the possible effects on the pregnancy. The available information is as follows:Acitretin, the active metabolite of etretinate, is teratogenic and is contraindicated during pregnancy. The risk of severe fetal malformations is well established when systemic retinoids are taken during pregnancy. Pregnancy must also be prevented after stopping acitretin therapy, while the drug is being eliminated to below a threshold blood concentration that would be associated with an increased incidence of birth defects. Because this threshold has not been established for acitretin in humans and because elimination rates vary among patients, the duration of posttherapy contraception to achieve adequate elimination cannot be calculated precisely. It is strongly recommended that contraception be continued for at least 3 years after stopping treatment with acitretin, based on the following considerations:
o In the absence of transesterification to form etretinate, greater than 98% of the acitretin would be eliminated within 2 months, assuming a mean elimination half-life of 49 hours.
o In cases where etretinate is formed, as has been demonstrated with concomitant administration of acitretin and ethanol,
♦ greater than 98% of the etretinate formed would be eliminated in 2 years, assuming a mean elimination half-life of 120 days.
♦ greater than 98% of the etretinate formed would be eliminated in 3 years, based on the longest demonstrated elimination half-life of 168 days. However, etretinate was found in plasma and subcutaneous fat in one patient reported to have had sporadic alcohol intake, 52 months after she stopped acitretin therapy.2• Severe birth defects have been reported where conception occurred during the time interval when the patient was being treated with acitretin and/or etretinate. In addition, severe birth defects have also been reported when conception occurred after the mother completed therapy. These cases have been reported both prospectively (before the outcome was known) and retrospectively (after the outcome was known). The events below are listed without distinction as to whether the reported birth defects are consistent with retinoid-induced embryopathy or not.
♦ There have been 318 prospectively reported cases involving pregnancies and the use of etretinate, acitretin, or both. In 238 of these cases, the conception occurred after the last dose of etretinate (103 cases), acitretin (126), or both (9). Fetal outcome remained unknown in approximately one-half of these cases, of which 62 were terminated and 14 were spontaneous abortions. Fetal outcome is known for the other 118 cases and 15 of the outcomes were abnormal (including cases of absent hand/wrist, clubfoot, GI malformation, hypocalcemia, hypotonia, limb malformation, neonatal apnea/anemia, neonatal ichthyosis, placental disorder/death, undescended testicle, and 5 cases of premature birth). In the 126 prospectively reported cases where conception occurred after the last dose of acitretin only, 43 cases involved conception at least 1 year but less than 2 years after the last dose. There were 3 reports of abnormal outcomes out of these 43 cases (involving limb malformation, GI tract malformations, and premature birth). There were only 4 cases where conception occurred at least 2 years after the last dose but there were no reports of birth defects in these cases.
♦ There is also a total of 35 retrospectively reported cases where conception occurred at least 1 year after the last dose of etretinate, acitretin, or both. From these cases there are 3 reports of birth defects when the conception occurred at least 1 year but less than 2 years after the last dose of acitretin (including heart malformations, Turner’s Syndrome, and unspecified congenital malformations) and 4 reports of birth defects when conception occurred 2 or more years after the last dose of acitretin (including foot malformation, cardiac malformations [2 cases], and unspecified neonatal and infancy disorder). There were 3 additional abnormal outcomes in cases where conception occurred 2 or more years after the last dose of etretinate (including chromosome disorder, forearm aplasia, and stillbirth).
♦ Females who have taken TEGISON (etretinate) must continue to follow the contraceptive recommendations for TEGISON. TEGISON is no longer marketed in the US; for information, call Alembic Pharmaceuticals Limited at 1-866-210-9797.
♦ Patients should not donate blood during and for at least 3 years following the completion of therapy with acitretin because women of childbearing potential must not receive blood from patients being treated with acitretin.
Important Information for Males Taking Acitretin:
Patients should not donate blood during and for at least 3 years following therapy with acitretin because women of childbearing potential must not receive blood from patients being treated with acitretin.• Samples of seminal fluid from 3 male patients treated with acitretin and 6 male patient treated with etretinate have been assayed for the presence of acitretin. The maximum concentration of acitretin observed in the seminal fluid of these men was 12.5 ng per mL. Assuming an ejaculate volume of 10 mL, the amount of drug transferred in semen would be 125 ng, which is 1/200,000 of a single 25-mg capsule. Thus, although it appears that residual acitretin in seminal fluid poses little, if any, risk to a fetus while a male patient is taking the drug or after it is discontinued, the no-effect limit for teratogenicity is unknown and there is no registry for birth defects associated with acitretin. The available data are as follows:
There have been 25 cases of reported conception when the male partner was taking acitretin. The pregnancy outcome is known in 13 of these 25 cases. Of these, 9 reports were retrospective and 4 were prospective (meaning the pregnancy was reported prior to knowledge of the outcome)3.
Timing of Paternal Acitretin Treatment Relative to Conception
Delivery of Healthy Neonate
Spontaneous Abortion
Induced Abortion
Total
At time of conception
5a
5
1
11
Discontinued ~4 weeks prior
0
0
1b
1
Discontinued ~6 to 8 months prior
0
1
0
1
a Four of 5 cases were prospective
b With malformation pattern not typical of retinoid embryopathy (bilateral cystic hygromas of neck. hypoplasia of lungs bilateral. VSD with overriding truncus arteriosus).For All Patients: AN ACITRETIN MEDICATION GUIDE MUST BE GIVEN TO THE PATIENT EACH TIME ACITRETIN IS DISPENSED, AS REQUIRED BY LAW.
Close -
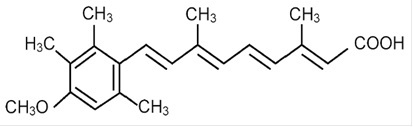
DESCRIPTIONAcitretin, USP a retinoid, is available in 10-mg, 17.5-mg, and 25-mg gelatin capsules for oral administration. Chemically, acitretin is ...
-
CLINICAL PHARMACOLOGYThe mechanism of action of acitretin is unknown. Pharmacokinetics: Absorption: Oral absorption of acitretin is optimal when given with food. For this reason, acitretin was given with food in all ...
-
INDICATIONS AND USAGEAcitretin capsules are indicated for the treatment of severe psoriasis in adults. Because of significant adverse effects associated with its use, acitretin capsules should be prescribed only by ...
-
CONTRAINDICATIONSPregnancy : See boxed CONTRAINDICATIONS AND WARNINGS. Acitretin is contraindicated in patients with severely impaired liver or kidney function and in patients with chronic abnormally elevated ...
-
WARNINGS(See also boxed CONTRAINDICATIONS AND WARNINGS.) Hepatotoxicity: Of the 525 subjects treated in US clinical trials, 2 had clinical jaundice with elevated serum bilirubin and transaminases ...
-
PRECAUTIONSA description of the H.A.E.R.T. materials is provided below. The main goals of the materials are to explain the program requirements, to reinforce the educational messages, and to assess program ...
-
ADVERSE REACTIONSHypervitaminosis A produces a wide spectrum of signs and symptoms primarily of the mucocutaneous, musculoskeletal, hepatic, neuropsychiatric, and central nervous systems. Many of the clinical ...
-
OVERDOSAGEIn the event of acute overdosage, acitretin must be withdrawn at once. Symptoms of overdose are identical to acute hypervitaminosis A (e.g., headache and vertigo). The acute oral toxicity (LD50 ...
-
DOSAGE AND ADMINISTRATIONThere is intersubject variation in the pharmacokinetics, clinical efficacy, and incidence of side effects with acitretin capsules. A number of the more common side effects are dose-related ...
-
HOW SUPPLIEDAcitretin capsules, USP are available as follows: 10 mg, opaque brown cap / opaque white body hard gelatin capsules size “4” having imprinting “A” on cap and “232” on body with black ink filled ...
-
REFERENCES1. Berbis Ph, et al.: Arch Dermatol Res (1988) 280:388-389. 2. Maier H, Honigsmann H: Concentration of etretinate in plasma and subcutaneous fat after long-term acitretin. Lancet 348:1107 ...
-
PATIENT AGREEMENT/INFORMED CONSENT FOR FEMALE PATIENTSTo be completed by the patient* and signed by her prescriber - *Must also be initialed by the parent or guardian of a minor patient (under age 18) Read each item below and initial in the ...
-
MEDICATION GUIDEMEDICATION GUIDE - Acitretin (A si TRE tin) Capsules, USP - Read this Medication Guide carefully before you start taking acitretin capsules and read it each time ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL -10 mgNDC 46708-741-30 - Acitretin - Capsules, USP - 10 mg - PHARMACIST: PROVIDE A MEDICATION GUIDE WITH - EACH PRESCRIPTION. DO NOT COVER LOT AND EXPIRY. Rx only - 30 Capsules - Alembic
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 17.5 mgNDC 46708-742-30 - Acitretin - Capsules, USP - 17.5 mg - PHARMACIST: PROVIDE A MEDICATION GUIDE WITH - EACH PRESCRIPTION. DO NOT COVER LOT AND EXPIRY. Rx only - 30 Capsules - Alembic
-
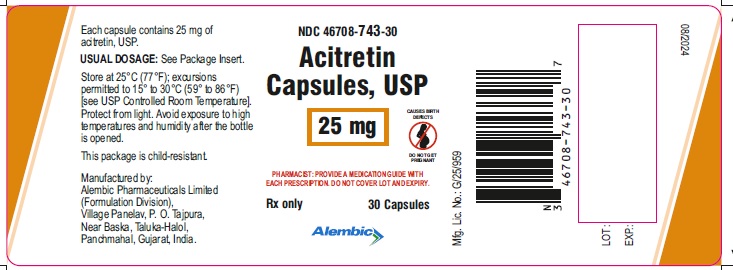
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 25 mgNDC 646708-743-30 - Acitretin - Capsules, USP - 25 mg - PHARMACIST: PROVIDE A MEDICATION GUIDE WITH - EACH PRESCRIPTION. DO NOT COVER LOT AND EXPIRY. Rx only - 30 Capsules - Alembic
-
INGREDIENTS AND APPEARANCEProduct Information