Label: DILTIAZEM HYDROCHLORIDE tablet
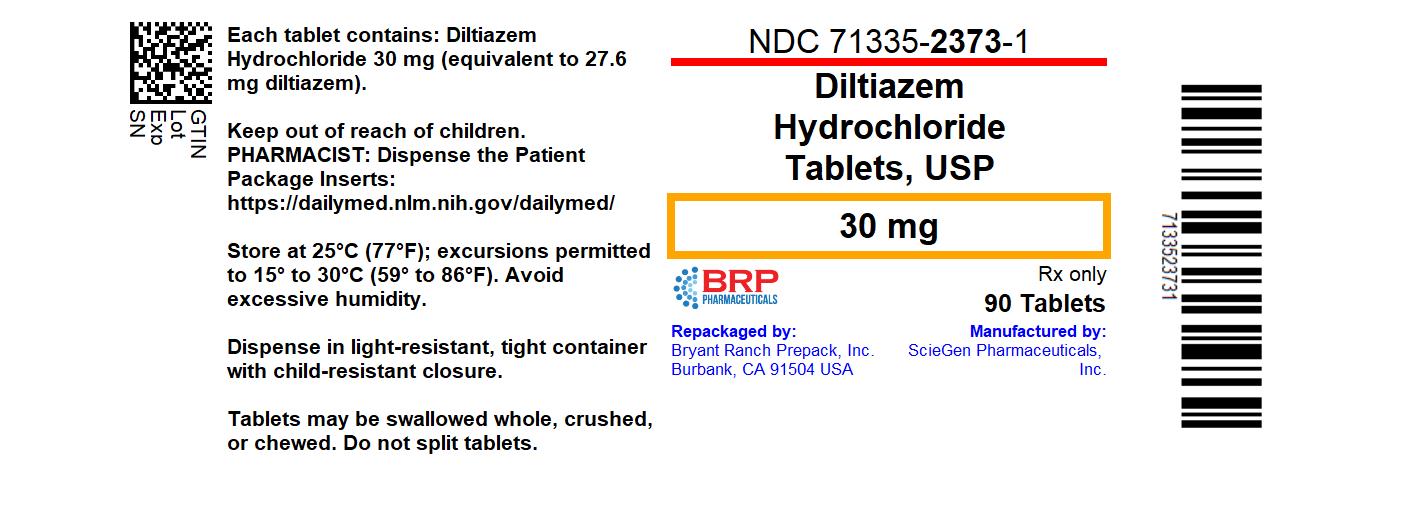
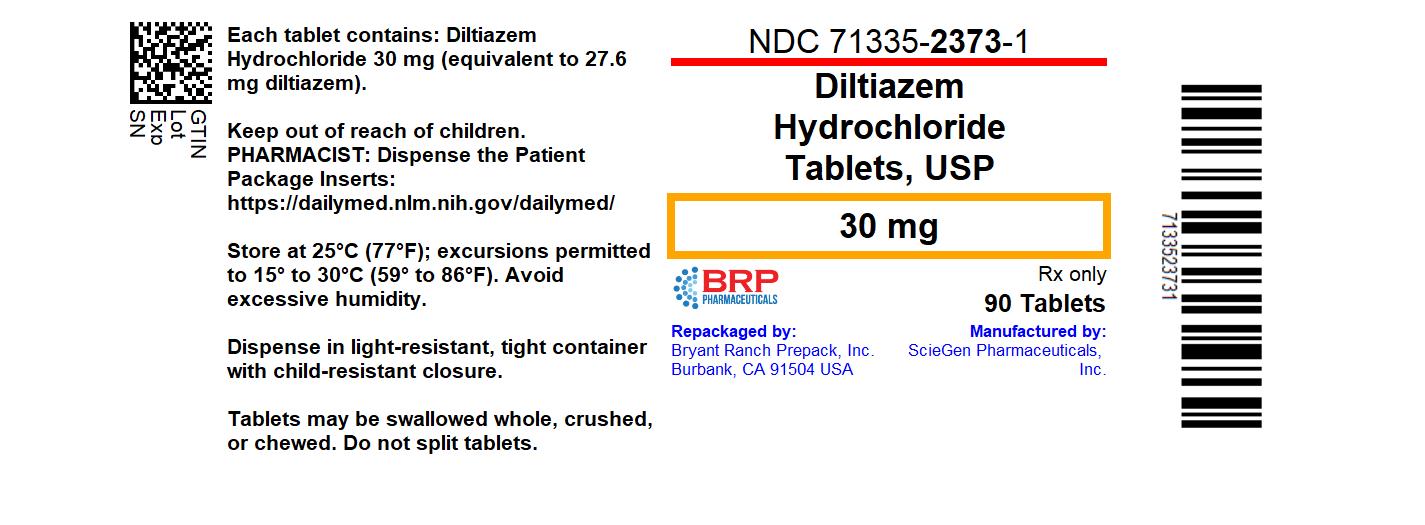
- NDC Code(s): 71335-2373-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 50228-481
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
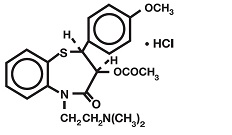
DESCRIPTIONDiltiazem hydrochloride, USP is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5 ...
-
CLINICAL PHARMACOLOGYThe therapeutic benefits achieved with diltiazem hydrochloride are believed to be related to its ability to inhibit the influx of calcium ions during membrane depolarization of cardiac and ...
-
INDICATIONS AND USAGEDiltiazem hydrochloride tablets are indicated for the management of chronic stable angina and angina due to coronary artery spasm.
-
CONTRAINDICATIONSDiltiazem hydrochloride tablets are contraindicated in: Patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker ...
-
WARNINGS1. Cardiac Conduction: Diltiazem hydrochloride prolongs AV node refractory periods without - significantly prolonging sinus node recovery time, except in patients with sick sinus syndrome. This ...
-
PRECAUTIONSGeneral - Diltiazem hydrochloride is extensively metabolized by the liver and excreted by the kidneys and in bile. As with any drug given over prolonged periods, laboratory parameters of renal ...
-
ADVERSE REACTIONSSerious adverse reactions have been rare in studies carried out to date, but it should be recognized that patients with impaired ventricular function and cardiac conduction abnormalities usually ...
-
OVERDOSAGEThe oral LD - 50s in mice and rats range from 415 mg/kg to 740 mg/kg and from 560 mg/kg to 810 mg/kg, respectively. The intravenous LD - 50s in these species were 60 ...
-
DOSAGE AND ADMINISTRATIONExertional Angina Pectoris Due to Atherosclerotic Coronary Artery Disease or Angina Pectoris at Rest Due to Coronary Artery Spasm: Dosage must be adjusted to each patient’s needs. Starting with ...
-
HOW SUPPLIEDDiltiazem hydrochloride tablets - Each white to off white, round shaped, film coated tablet is debossed with “481” on one side and plain on other side. NDC: 71335-2373-1: 90 TABLETs in a ...
-
PRINCIPAL DISPLAY PANELDiltiazem Hcl 30mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information