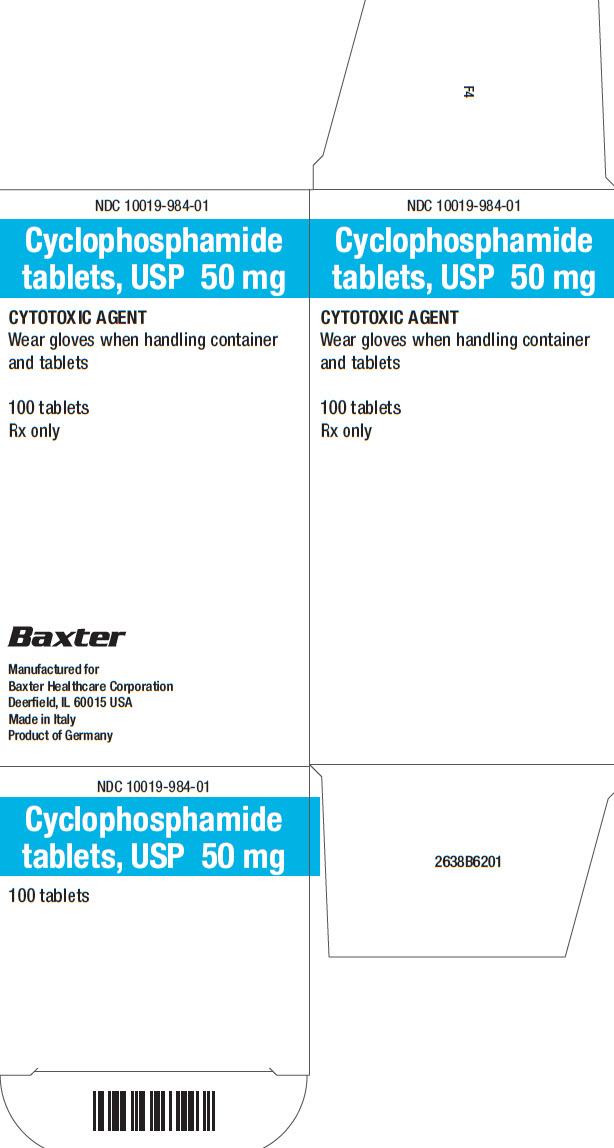
Label: CYCLOPHOSPHAMIDE tablet
- NDC Code(s): 10019-982-01, 10019-982-09, 10019-984-01, 10019-984-09
- Packager: Baxter Healthcare Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 7, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CYCLOPHOSPHAMIDE TABLETS safely and effectively. See full prescribing information for CYCLOPHOSPHAMIDE TABLETS. CYCLOPHOSPHAMIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE 1.1 Malignant Diseases - Cyclophosphamide Tablets is indicated for the treatment of: • malignant lymphomas (Stages III and IV of the Ann Arbor staging system), Hodgkin’s disease, lymphocytic ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Hydration and Important Administration Instructions - During or immediately after the administration of Cyclophosphamide Tablets, adequate amounts of fluid should be ingested or infused to ...
-
3 DOSAGE FORMS AND STRENGTHS Tablets: • 25 mg of cyclophosphamide, USP. Tablets are white with blue flecks and imprinted with 25 on one side and BXT on the other. • 50 mg of cyclophosphamide, USP. Tablets are white with ...
-
4 CONTRAINDICATIONS Cyclophosphamide Tablets are contraindicated in patients with: • A history of severe hypersensitivity reactions to cyclophosphamide, any of its metabolites, or to other components of the product ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Myelosuppression, Immunosuppression, Bone Marrow Failure and Infections - Cyclophosphamide can cause myelosuppression (leukopenia, neutropenia, thrombocytopenia and anemia), bone marrow ...
-
6 ADVERSE REACTIONS The following adverse reactions are discussed in more detail in other sections of the labeling. • Hypersensitivity [see Contraindications (4)] • Myelosuppression, Immunosuppression, Bone Marrow ...
-
7 DRUG INTERACTIONS Cyclophosphamide is a pro-drug that is activated by cytochrome P450s [see Clinical Pharmacology (12.3)]. An increase of the concentration of cytotoxic metabolites may occur with: • Protease ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on its mechanism of action and published reports of effects in pregnant patients or animals, Cyclophosphamide Tablets can cause fetal harm when administered ...
-
10 OVERDOSAGE No specific antidote for cyclophosphamide is known. Overdosage should be managed with supportive measures, including appropriate treatment for any concurrent infection, myelosuppression, or ...
-
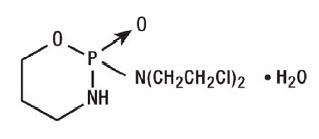
11 DESCRIPTION Cyclophosphamide is a synthetic antineoplastic drug chemically related to the nitrogen mustards. The chemical name for cyclophosphamide is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - The mechanism of action is thought to involve cross-linking of tumor cell DNA. 12.2 Pharmacodynamics - Cyclophosphamide is biotransformed principally in the liver ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Cyclophosphamide administered by different routes, including intravenous, subcutaneous or intraperitoneal injection, or in drinking ...
-
15 REFERENCES 1. OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
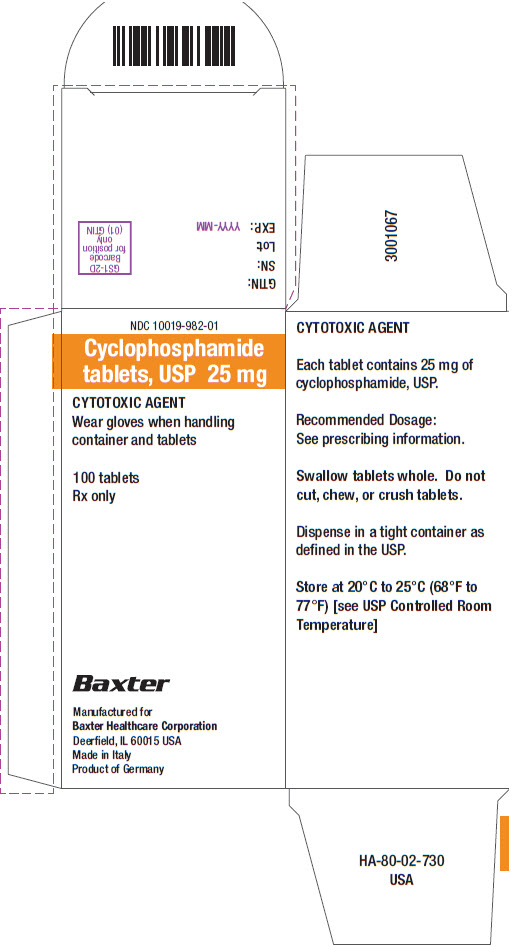
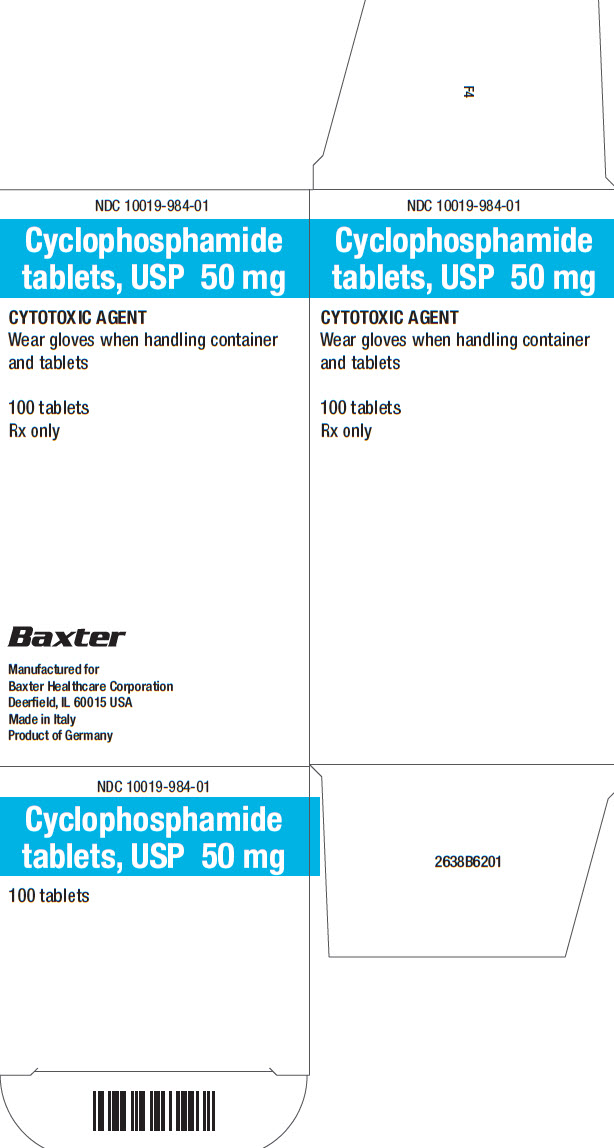
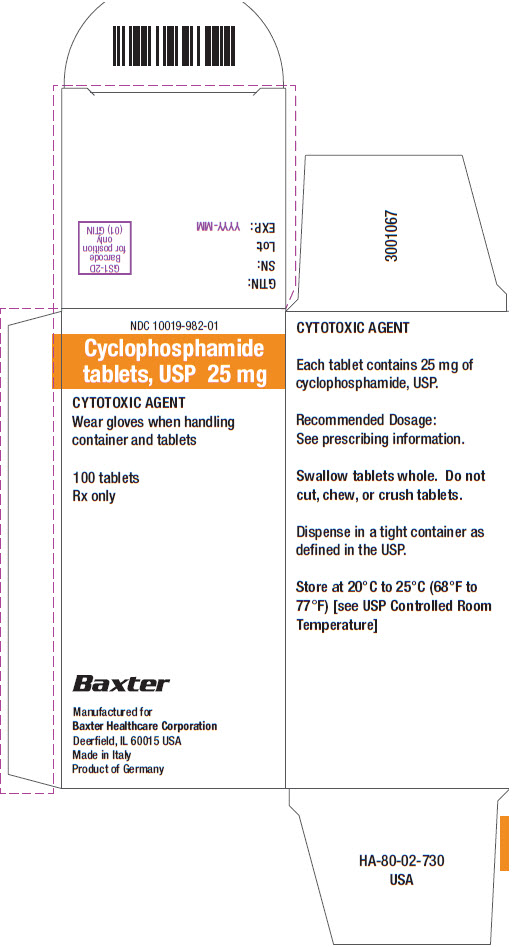
16 HOW SUPPLIED/STORAGE AND HANDLING Tablets: • 25 mg of cyclophosphamide, USP. Tablets are white with blue flecks and imprinted with 25 on one side and BXT on the other side. NDC 10019-982-01: Carton containing bottle of 100 ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient of the following: Myelosuppression, Immunosuppression, and Infections - • Inform patients of the possibility of myelosuppression, immunosuppression, and infections. Explain the ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Container Label - Barcode - 100 Tablets - NDC 10019-982-09 - Cyclophosphamide - tablets, USP 25 mg - CYTOTOXIC AGENT - Wear gloves when handling container - and tablets - Rx only - Baxter ...
-
INGREDIENTS AND APPEARANCEProduct Information