Label: CLOBETASOL PROPIONATE suspension/ drops
- NDC Code(s): 81046-0319-1
- Packager: Eyenovia, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BYQLOVI - ®safely and effectively. See full prescribing information for BYQLOVI. BYQLOVI, for topical ophthalmic use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBYQLOVI is indicated for the treatment of post-operative inflammation and pain following ocular surgery.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Instill one drop of BYQLOVI into the affected eye twice daily beginning the day after surgery and continuing throughout the first 2 weeks of the post-operative ...
-
3 DOSAGE FORMS AND STRENGTHSOphthalmic suspension containing clobetasol propionate 0.05% (0.5 mg/mL).
-
4 CONTRAINDICATIONSBYQLOVI is contraindicated in most active viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in ...
-
5 WARNINGS AND PRECAUTIONS5.1 Intraocular Pressure (IOP) Increase - Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be ...
-
6 ADVERSE REACTIONSThe following serious reactions are found elsewhere in the labeling: Intraocular Pressure (IOP) Increase - [see - Warnings and Precautions (5.1)] Posterior Subcapsular Cataract ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled clinical studies of BYQLOVI administration in pregnant women to inform drug-associated risks. Plasma concentrations of ...
-
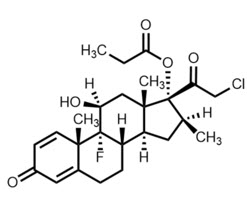
11 DESCRIPTIONBYQLOVI (clobetasol propionate ophthalmic suspension) 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, that has a high degree of glucocorticoid activity and a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Like other topical corticosteroids, clobetasol propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term animal studies have not been performed with BYQLOVI to evaluate the carcinogenic potential of clobetasol ...
-
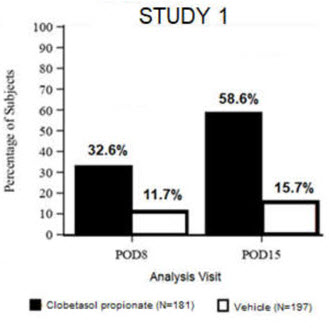
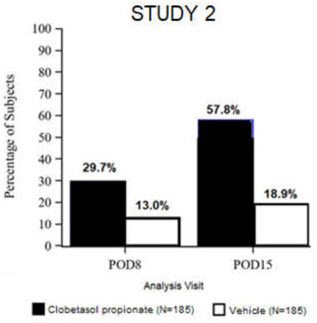
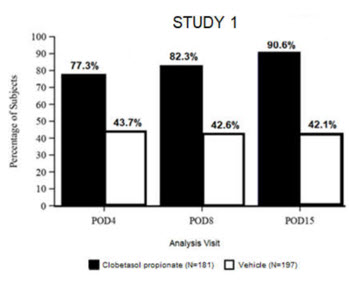
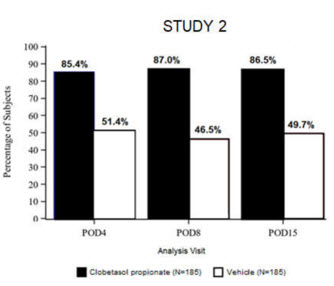
14 CLINICAL STUDIESClinical efficacy was evaluated in 2 multi-center, randomized, double-masked, vehicle-controlled trials in which patients had ≥10 cells in the anterior chamber after cataract surgery were assigned ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - BYQLOVI (clobetasol propionate ophthalmic suspension) 0.05% (0.5 mg/mL) is a sterile ophthalmic suspension. It is supplied in a multi-dose white low-density polyethylene ...
-
17 PATIENT COUNSELING INFORMATIONAdministration with Other Eye Drops - Advise patients to wait at least 5 minutes between instillation of BYQLOVI and other eye drops if using other eye drops in addition to BYQLOVI. Risk ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Eyenovia, Inc. 295 Madison Avenue, Suite 2400 - New York, NY 10017
-
PRINCIPAL DISPLAY PANEL - 3.5 mL Bottle and Carton LabelsNDC #: 81046-0319-1 - BYQLOVI - (clobetasol propionate - ophthalmic suspension) 0.05% FOR TOPICAL - APPLICATION IN THE EYE - Sterile - Rx only - 3.5 mL - eyenovia - Bottle Label - Carton ...
-
INGREDIENTS AND APPEARANCEProduct Information