Label: TARGET DYE FREE CHILDRENS ALLERGY RELIEF- diphenhydramine hydrochloride syrup
- NDC Code(s): 82442-710-08
- Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Do not use
- ▪
- to make a child sleepy
- ▪
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if the child has
- ▪
- a breathing problem such as chronic bronchitis
- ▪
- glaucoma
-
Directions
- ▪
- find right dose on chart below
- ▪
- take every 4 to 6 hours, or as directed by a doctor
- ▪
- do not take more than 6 doses in 24 hours
- ▪
- mL = milliliter
Age (yr)
Dose (tsp)
children under 2 years
do not use
children 2 to 5 years
do not use unless directed by a doctor
children 6 to 11 years
5 mL to 10 mL
Attention: use only enclosed dosing cup designed for use with this product. Do not use any other dosing device.
- Other information
- Inactive ingredients
-
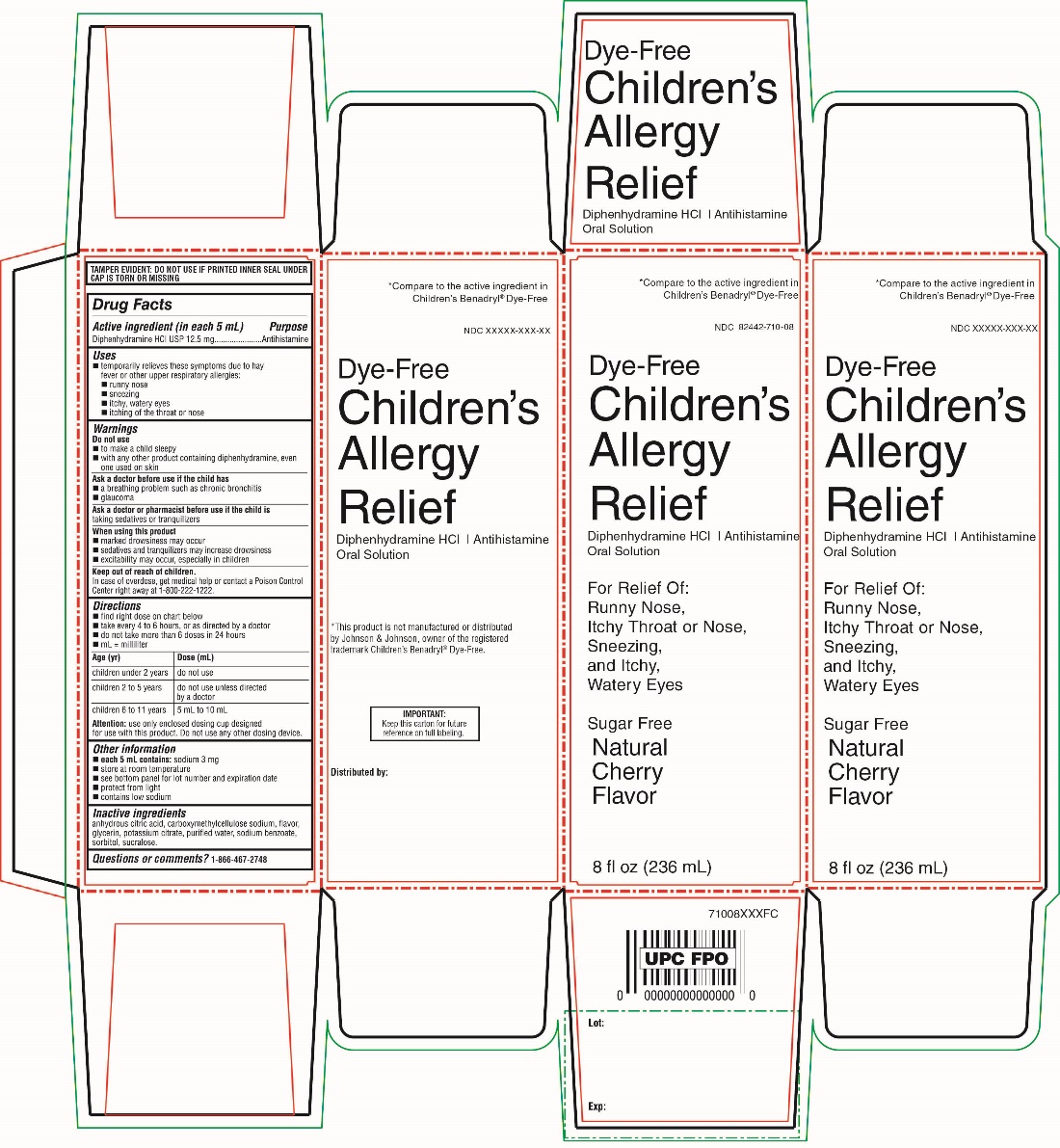
PRINCIPAL DISPLAY PANEL
NDC 82442-710-08
Compare to active ingredient in Children's
Benadryl® Dye-FreeDye-Free
Children’s Allergy Relief
Diphenhydramine HCl / Antihistamine
Oral solution
For Relief of:
- Runny nose
- Itchy throat or Nose
- sneezing
- itchy, watery eyes
Sugar Free
Natural Cherry Flavor
8 fl oz (236 mL)*This product is not manufactured or distributed by Johnson & Johnson, owner of the registered trademark Children’s Benadryl® Dye –Free.
IMPORTANT: Keep this carton for future reference on full labelling.
TAMPER EVIDENT: DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS TORN OR MISSING
Distributed by:
-
INGREDIENTS AND APPEARANCE
TARGET DYE FREE CHILDRENS ALLERGY RELIEF
diphenhydramine hydrochloride syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82442-710 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Diphenhydramine hydrochloride (UNII: TC2D6JAD40) (diphenhydramine - UNII:8GTS82S83M) Diphenhydramine hydrochloride 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) glycerin (UNII: PDC6A3C0OX) POTASSIUM CITRATE (UNII: EE90ONI6FF) water (UNII: 059QF0KO0R) sodium benzoate (UNII: OJ245FE5EU) sorbitol (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color RED (Clear, colorless) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82442-710-08 1 in 1 CARTON 04/05/2024 1 236 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/05/2024 Labeler - Target Corporation (006961700)