Label: ERGOCALCIFEROL CAPSULES, capsule
- NDC Code(s): 70518-3472-0, 70518-3472-1
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 42806-547
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 2, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
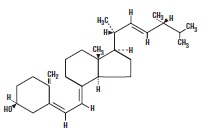
Ergocalciferol Capsules, USP is a synthetic calcium regulator for oral administration. Ergocalciferol is a white, colorless crystal, insoluble in water, soluble in organic solvents, and slightly ...
-
CLINICAL PHARMACOLOGYThe - in vivosynthesis of the major biologically active metabolites of vitamin D occurs in two steps. The first hydroxylation of ergocalciferol takes place in the liver (to 25-hydroxyvitamin D ...
-
INDICATIONS AND USAGEErgocalciferol Capsules are indicated for use in the treatment of hypoparathyroidism, refractory rickets, also known as vitamin D resistant rickets, and familial hypophosphatemia.
-
CONTRAINDICATIONSErgocalciferol Capsules are contraindicated in patients with hypercalcemia, malabsorption syndrome, abnormal sensitivity to the toxic effects of vitamin D, and hypervitaminosis D.
-
WARNINGSHypersensitivity to vitamin D may be one etiologic factor in infants with idiopathic hypercalcemia. In these cases vitamin D must be strictly restricted. Keep out of the reach of children.
-
PRCAUTIONSGeneral - Vitamin D administration from fortified foods, dietarysupplements, self-administered and prescription drug sources should be evaluated. Therapeutic dosage should be readjusted as soon ...
-
ADVERSE REACTIONSHypervitaminosis D is characterized by effects on the following organ system: Renal:Impairment of renal function with polyuria, nocturia, polydipsia, hypercalciuria, reversible azotemia ...
-
OVERDOSAGEThe effects of administered vitamin D can persist for two or more months after cessation of treatment. Hypervitaminosis D is characterized by: 1. Hypercalcemia with anorexia, nausea, weakness ...
-
DOSAGE AND ADMINISTRATIONTHE RANGE BETWEEN THERAPEUTIC AND TOXIC DOSES IS NARROW. Vitamin D Resistant Rickets: 12,000 to 500,000 IU units daily. Hypoparathyroidism:50,000 to 200,000 IU units daily concomitantly with ...
-
HOW SUPPLIEDCapsules of 1.25 mg (50,000 IU vitamin D) of ergocalciferol, USP are clear green oval softgel capsules filled with a clear to pale yellow - NDC: 70518-3472-00 - NDC: 70518-3472-01 - PACKAGING: 4 in 1 ...
-
PRINCIPAL DISPLAY PANELDRUG: Ergocalciferol Capsules, GENERIC: Ergocalciferol Capsules, DOSAGE: CAPSULE - ADMINSTRATION: ORAL - NDC: 70518-3472-0 - NDC: 70518-3472-1 - COLOR: green - SHAPE: OVAL - SCORE: No score - SIZE: 13 ...
-
INGREDIENTS AND APPEARANCEProduct Information