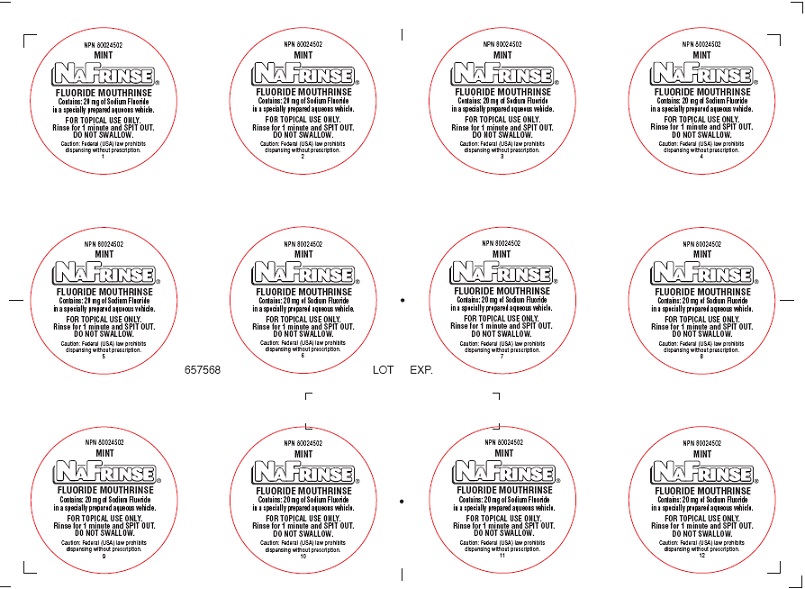
Label: NAFRINSE UNIT DOSE MINT FLAVOR- sodium fluoride and hydrofluoric acid solution
NAFRINSE UNIT DOSE BUBBLE GUM- sodium fluoride and hydrofluoric acid solution
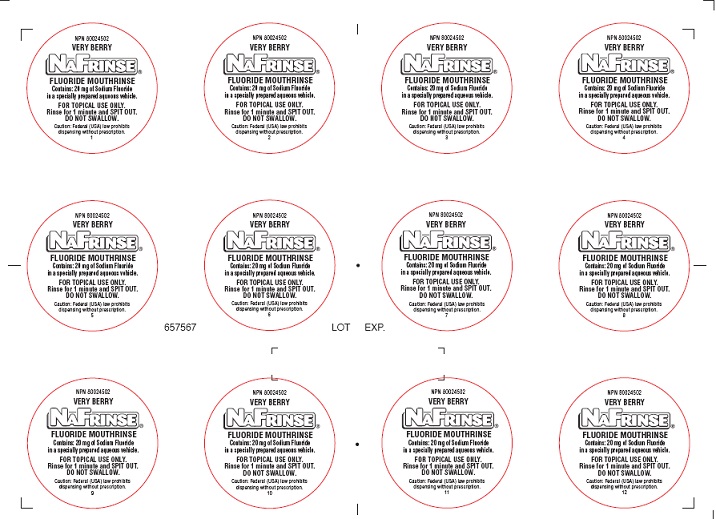
NAFRINSE UNIT DOSE VERRY BERRY- sodium fluoride and hydrofluoric acid solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0273-8002-01, 0273-8003-01, 0273-8004-01, 0273-8005-01 - Packager: Young Dental Manufacturing Co 1, LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 14, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Inactives
- Purpose
- Warning:
-
Dosage and Administration:
DIRECTIONS FOR USE: {Best Results • Use Early Morning)
1. Each pupil receives one cup and one napkin. 2. Have the pupils remove the lids from the cups. 3. Have the pupils empty the contents of the cup into their mouths and swish thoroughly for one minute. 4. HOLD CUP AGAINST THE MOUTH. Spit solution back into the cup. 5. Have the pupils wipe their mouths with the napkin; then stuff the napkin slowly into the cup to absorb the solution. 6. Discard the cup with the napkin into the provided trash bag. a) Do not swallow b) Do not eat, drink or rinse with water for 30 minutes after use. c) Instruct children under 12 years of age in the use of the product and in good brushing and rinsing habits to minimize swallowing d) Not recommended for use in children younger than 6 years of age. ADDITIONAL INSTRUCTIONS/IMPORTANT: In order to avoid spillage of the solution from the cup, the children must be instructed to do the following: 1) When spitting the fluoride solution from the mouth back into the cup, the children must hold the cup close to the mouth, and spit slowly into the cup. 2) The napkins must be stuffed into the cups very slowly in order to avoid spillage.
- Keep out of reach of children
- Product Label
-
INGREDIENTS AND APPEARANCE
NAFRINSE UNIT DOSE MINT FLAVOR
sodium fluoride and hydrofluoric acid solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0273-8002 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SACCHARIN SODIUM (UNII: SB8ZUX40TY) Potassium Sorbate (UNII: 1VPU26JZZ4) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Product Characteristics Color Score Shape Size Flavor MINT (mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0273-8002-01 10 mL in 1 CUP; Type 0: Not a Combination Product 08/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2017 NAFRINSE UNIT DOSE BUBBLE GUM
sodium fluoride and hydrofluoric acid solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0273-8003 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SACCHARIN SODIUM (UNII: SB8ZUX40TY) Potassium Sorbate (UNII: 1VPU26JZZ4) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Product Characteristics Color Score Shape Size Flavor BUBBLE GUM (bubble gum) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0273-8003-01 10 mL in 1 CUP; Type 0: Not a Combination Product 08/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2017 NAFRINSE UNIT DOSE BUBBLE GUM
sodium fluoride and hydrofluoric acid solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0273-8004 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SACCHARIN SODIUM (UNII: SB8ZUX40TY) Potassium Sorbate (UNII: 1VPU26JZZ4) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Product Characteristics Color Score Shape Size Flavor BUBBLE GUM (bubble gum) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0273-8004-01 5 mL in 1 CUP; Type 0: Not a Combination Product 08/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2017 NAFRINSE UNIT DOSE VERRY BERRY
sodium fluoride and hydrofluoric acid solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0273-8005 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SACCHARIN SODIUM (UNII: SB8ZUX40TY) Potassium Sorbate (UNII: 1VPU26JZZ4) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Product Characteristics Color Score Shape Size Flavor BERRY (very berry) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0273-8005-01 10 mL in 1 CUP; Type 0: Not a Combination Product 08/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2017 Labeler - Young Dental Manufacturing Co 1, LLC. (006309355) Registrant - Medical Products Laboratories, Inc (002290302) Establishment Name Address ID/FEI Business Operations Medical Products Laboratories, Inc 002290302 manufacture(0273-8002, 0273-8003, 0273-8004, 0273-8005)