Label: TRACLEER- bosentan tablet, film coated
TRACLEER- bosentan tablet, for suspension
- NDC Code(s): 66215-101-03, 66215-101-06, 66215-102-03, 66215-102-06, view more
- Packager: Actelion Pharmaceuticals US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRACLEER safely and effectively. See full prescribing information for TRACLEER. TRACLEER - ®(bosentan) tablets, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)Hepatotoxicity - In clinical studies, TRACLEER caused at least 3-fold upper limit of normal (ULN) elevation of liver aminotransferases (ALT and AST) in about 11% of patients, accompanied by ...
WARNING: RISKS OF HEPATOTOXICITY and EMBRYO-FETAL TOXICITY
Because of the risks of hepatotoxicity and birth defects, TRACLEER is available only through a restricted program called the Bosentan REMS Program. Under the Bosentan REMS, prescribers, patients, and pharmacies must enroll in the program [see Warnings and Precautions (5.3)] .
Hepatotoxicity
In clinical studies, TRACLEER caused at least 3-fold upper limit of normal (ULN) elevation of liver aminotransferases (ALT and AST) in about 11% of patients, accompanied by elevated bilirubin in a small number of cases. Because these changes are a marker for potential serious hepatotoxicity, serum aminotransferase levels must be measured prior to initiation of treatment and then monthly [see Dosage and Administration (2.4), Warnings and Precautions (5.1)] . In the postmarketing period, in the setting of close monitoring, rare cases of unexplained hepatic cirrhosis were reported after prolonged (>12 months) therapy with TRACLEER in patients with multiple comorbidities and drug therapies. There have also been reports of liver failure. The contribution of TRACLEER in these cases could not be excluded.
In at least one case, the initial presentation (after >20 months of treatment) included pronounced elevations in aminotransferases and bilirubin levels accompanied by non-specific symptoms, all of which resolved slowly over time after discontinuation of TRACLEER. This case reinforces the importance of strict adherence to the monthly monitoring schedule for the duration of treatment and the treatment algorithm, which includes stopping TRACLEER with a rise of aminotransferases accompanied by signs or symptoms of liver dysfunction [see Dosage and Administration (2.4)] .
Elevations in aminotransferases require close attention [see Dosage and Administration (2.4)] . TRACLEER should generally be avoided in patients with elevated aminotransferases (>3×ULN) at baseline because monitoring for hepatotoxicity may be more difficult. If liver aminotransferase elevations are accompanied by clinical symptoms of hepatotoxicity (such as nausea, vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin ≥2×ULN, treatment with TRACLEER should be stopped. There is no experience with the reintroduction of TRACLEER in these circumstances.
CloseEmbryo-Fetal Toxicity
TRACLEER is likely to cause major birth defects if used by pregnant females based on animal data [see Warnings and Precautions (5.2), Use in Specific Populations (8.1)] . Therefore, pregnancy must be excluded before the start of treatment with TRACLEER. Throughout treatment and for one month after stopping TRACLEER, females of reproductive potential must use two reliable methods of contraception unless the patient has an intrauterine device (IUD) or tubal sterilization, in which case no other contraception is needed. Hormonal contraceptives, including oral, injectable, transdermal, and implantable contraceptives should not be used as the sole means of contraception because these may not be effective in patients receiving TRACLEER [see Drug Interactions (7.2)] . Obtain monthly pregnancy tests.
-
1 INDICATIONS AND USAGETRACLEER is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1): in adults to improve exercise ability and to decrease clinical worsening. Studies establishing ...
-
2 DOSAGE AND ADMINISTRATION2.1 Required Monitoring - Healthcare professionals who prescribe TRACLEER must enroll in the Bosentan REMS Program and must comply with the required monitoring to minimize the risks associated ...
-
3 DOSAGE FORMS AND STRENGTHS62.5 mg tablets: round, biconvex, orange-white tablets, debossed with identification marking "62,5" 125 mg tablets: oval, biconvex, orange-white tablets, debossed with identification marking ...
-
4 CONTRAINDICATIONS4.1 Pregnancy - Use of TRACLEER is contraindicated in females who are or may become pregnant. To prevent pregnancy, females of reproductive potential must use two reliable forms of contraception ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity - ALT or AST >3 - ×ULN were observed in 11% of TRACLEER-treated patients (n=658) compared to 2% of placebo-treated patients (n=280). Three-fold increases were seen in 12% of 95 ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are described elsewhere in the labeling: Hepatotoxicity - [see - Boxed Warning, Warnings and Precautions (5.1)] Embryo-fetal ...
-
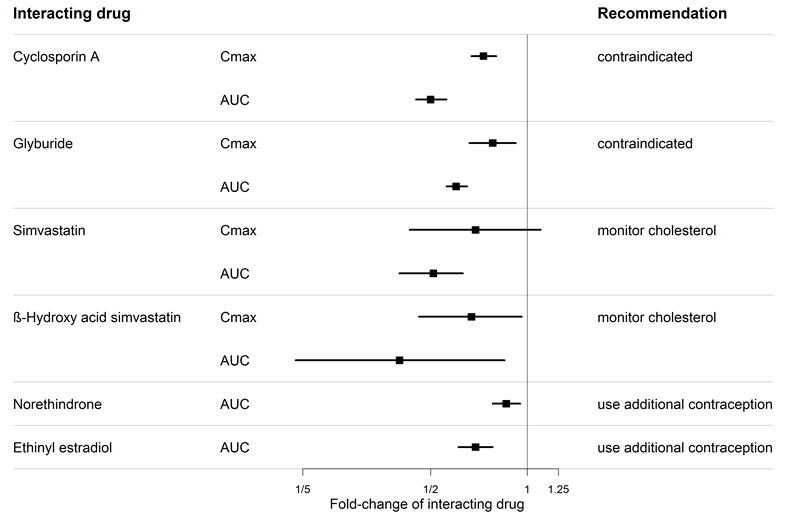
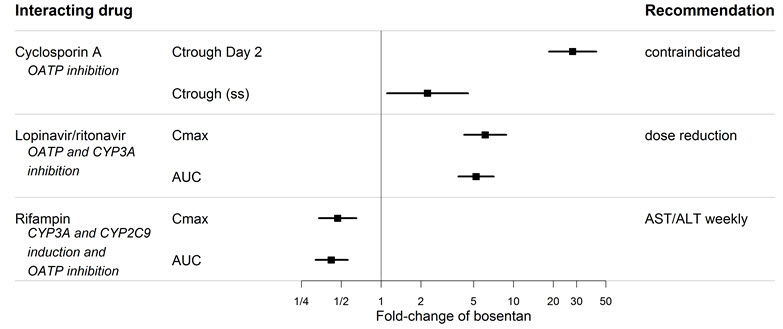
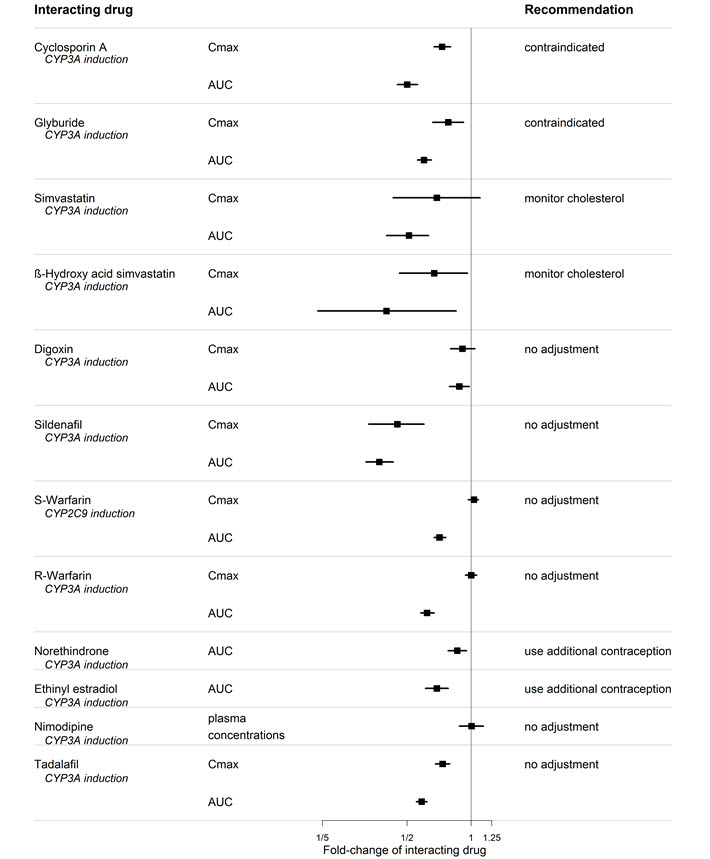
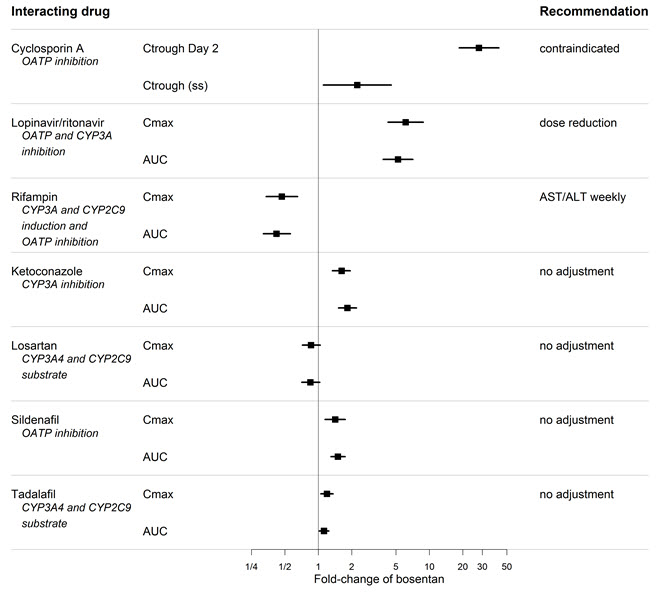
7 DRUG INTERACTIONS7.1 Cytochrome P450 Drug Interactions - Bosentan is metabolized by CYP2C9 and CYP3A. Inhibition of these enzymes may increase the plasma concentration of bosentan - [see - Pharmacokinetics ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on data from animal reproduction studies, TRACLEER may cause fetal harm, including birth defects and fetal death, when administered to a pregnant female ...
-
10 OVERDOSAGEBosentan has been given as a single dose of up to 2400 mg in normal volunteers, or up to 2000 mg/day for 2 months in patients, without any major clinical consequences. The most common side effect ...
-
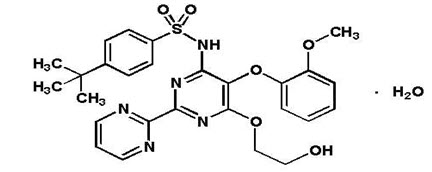
11 DESCRIPTIONTRACLEER - ® is the proprietary name for bosentan, an endothelin receptor antagonist that belongs to a class of highly substituted pyrimidine derivatives, with no chiral centers. It is designated ...
-
12 CLINICAL PHARMACOLOGY12.3 Pharmacokinetics - General - After oral administration, maximum plasma concentrations of bosentan are attained within 3–5 hours and the terminal elimination half-life is about 5 hours in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis and Mutagenesis - Two years of dietary administration of bosentan to mice produced an increased incidence of ...
-
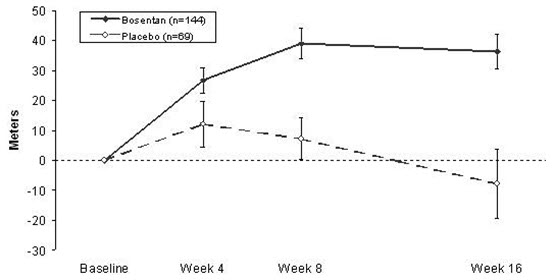
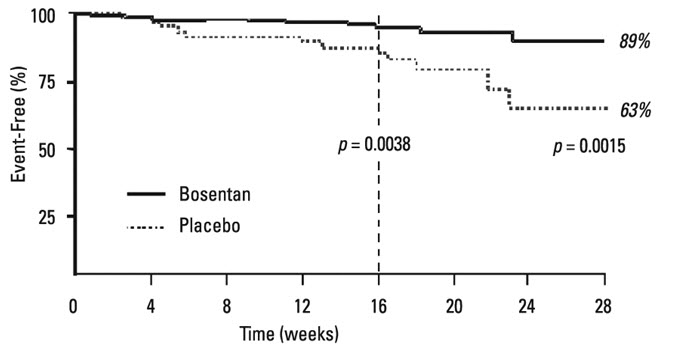
14 CLINICAL STUDIES14.1 Pulmonary Arterial Hypertension - WHO Functional Class III-IV - Two randomized, double-blind, multi-center, placebo-controlled trials were conducted in 32 and 213 patients. The larger ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING62.5 mg film-coated, round, biconvex, orange-white tablets, debossed with identification marking "62,5", packaged in a white high-density polyethylene bottle and a white polypropylene ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide) Restricted access - Advise the patient that TRACLEER is only available through a restricted access program ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Actelion Pharmaceuticals US, Inc. a Janssen Pharmaceutical Company - Titusville, NJ 08560, USA - For patent information: www.janssenpatents.com - © 2001 – 2019 ...
-
MEDICATION GUIDEMedication Guide - TRACLEER - ® (TRA-KLEER) (BOSENTAN) TABLETS - This Medication Guide has been approved by the U.S. Food and Drug ...
-
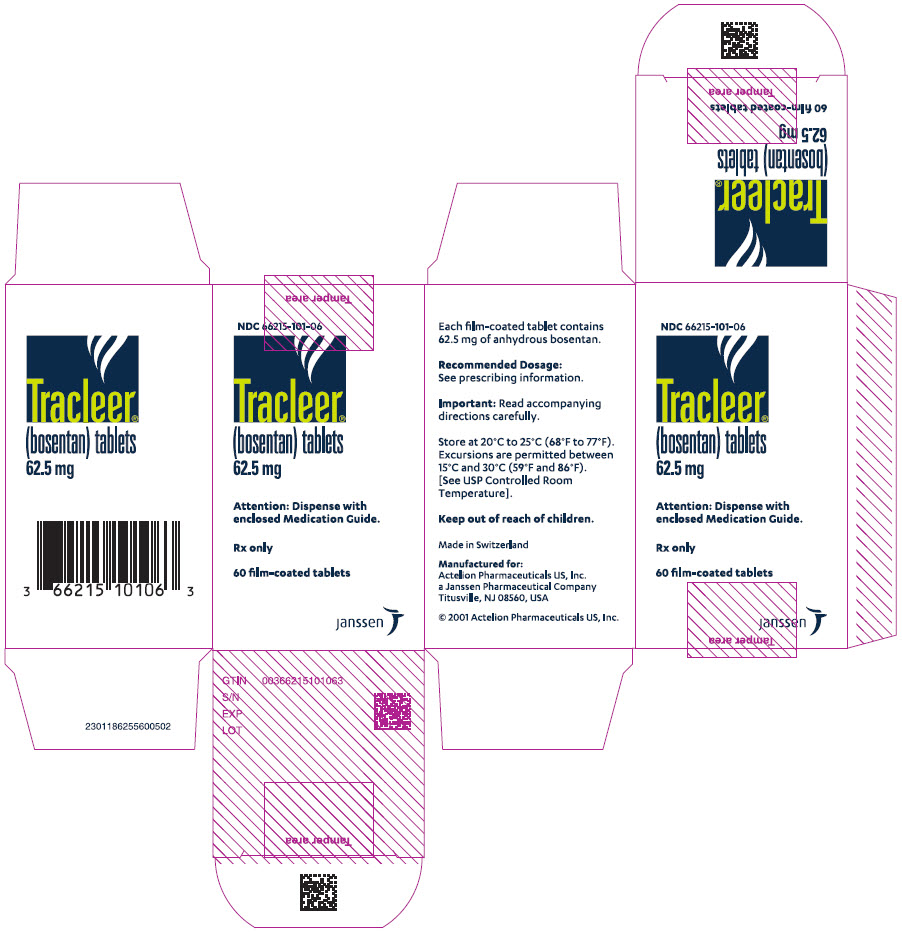
PRINCIPAL DISPLAY PANEL - 62.5 mg Tablet Bottle CartonNDC 66215-101-06 - Tracleer - ® (bosentan) tablets - 62.5 mg - Attention: Dispense with - enclosed Medication Guide. Rx only - 60 film-coated tablets - janssen
-
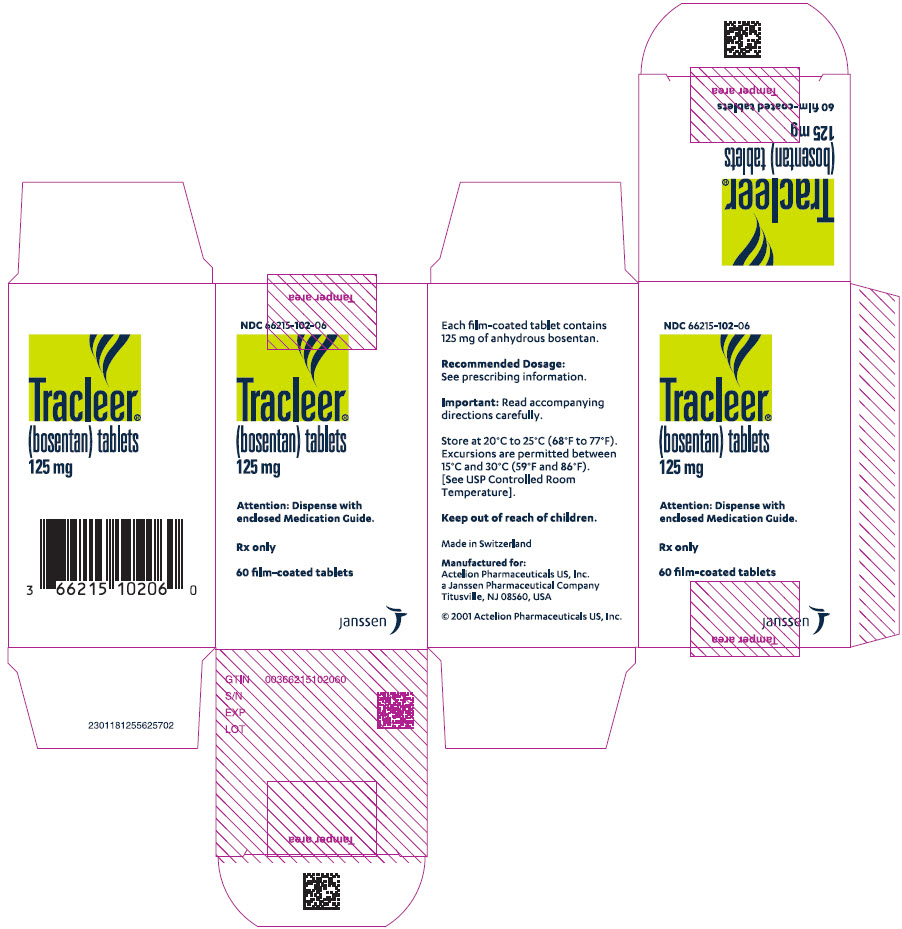
PRINCIPAL DISPLAY PANEL - 125 mg Tablet Bottle CartonNDC 66215-102-06 - Tracleer - ® (bosentan) tablets - 125 mg - Attention: Dispense with - enclosed Medication Guide. Rx only - 60 film-coated tablets - janssen
-
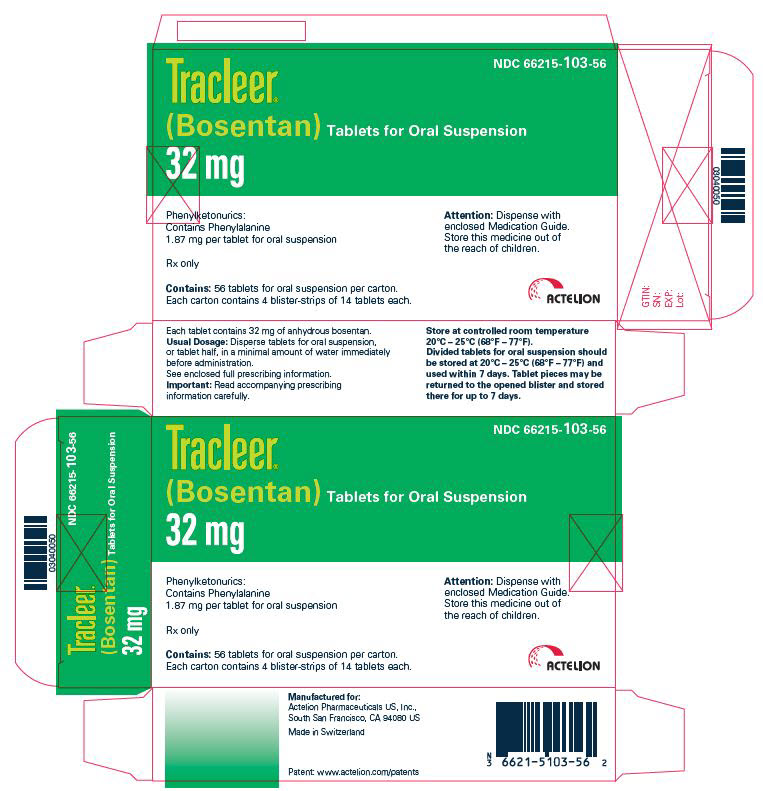
PRINCIPAL DISPLAY PANEL - 32 mg Tablet Blister Pack CartonNDC 66215-103-56 - TRACLEER - ® (Bosentan) Tablets for Oral Suspension - 32 mg - Phenylketonurics: Contains Phenylalanine - 1.87 mg per tablet for oral suspension - Rx ...
-
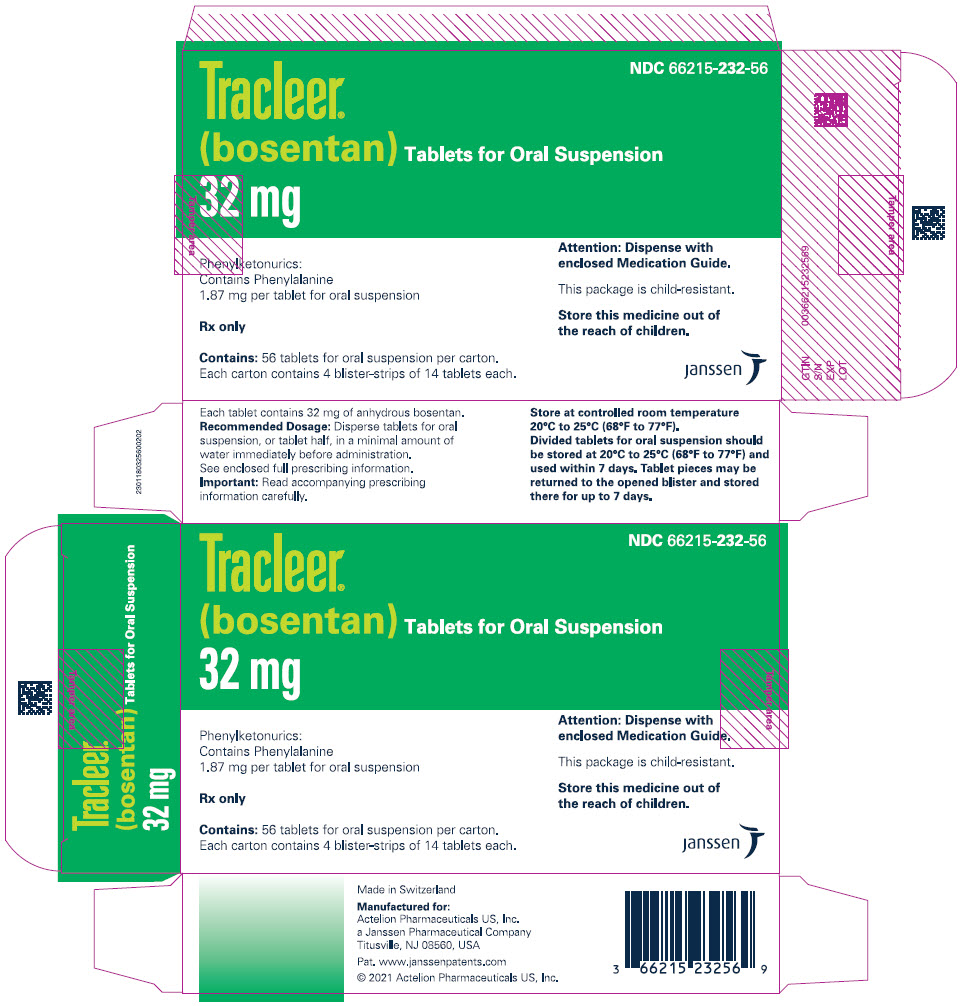
PRINCIPAL DISPLAY PANEL - 32 mg Tablet Blister Pack Carton - 232NDC 66215-232-56 - Tracleer - ® (bosentan) Tablets for Oral Suspension - 32 mg - Phenylketonurics: Contains Phenylalanine - 1.87 mg per tablet for oral suspension - Rx only - Contains ...
-
INGREDIENTS AND APPEARANCEProduct Information