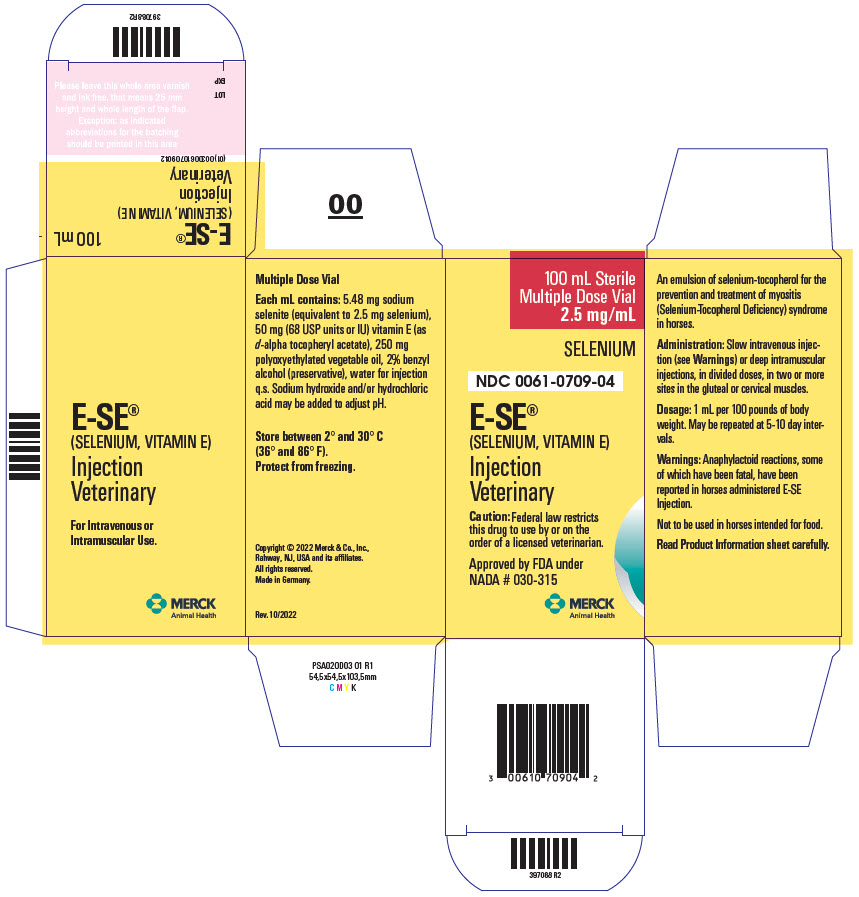
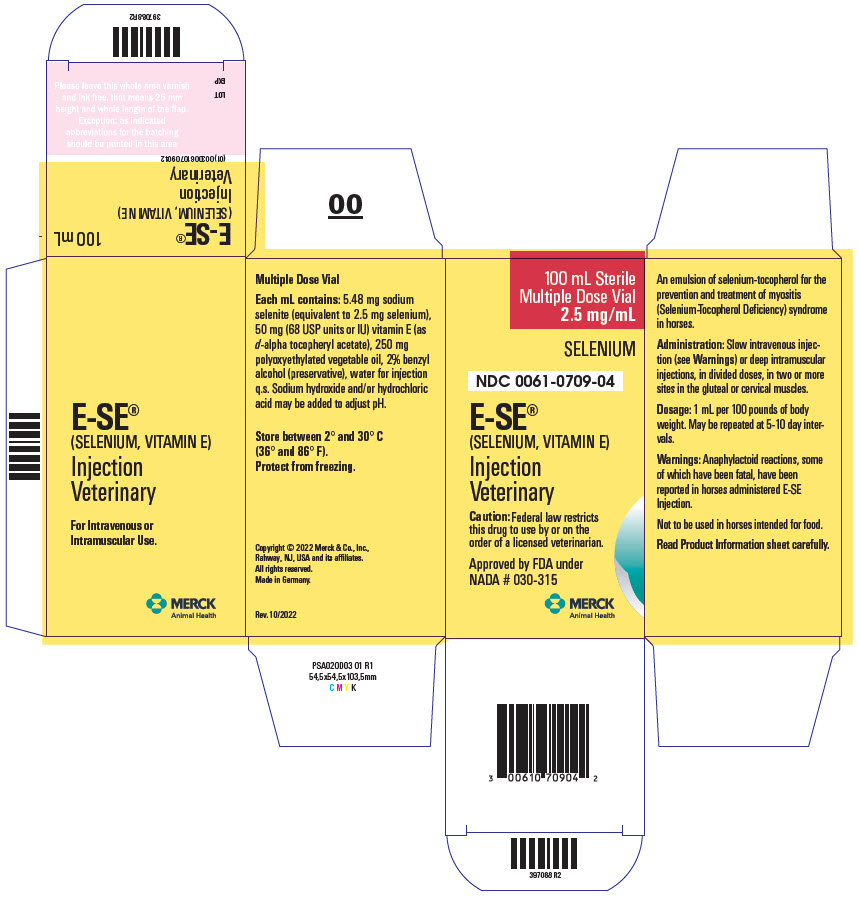
Label: E-SE- sodium selenite and .alpha.-tocopherol acetate, d- injection, solution
- NDC Code(s): 0061-0709-04
- Packager: Schering Corporation
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION E-SE Injection is an emulsion of selenium-tocopherol for the prevention and treatment of myositis (Selenium-Tocopherol Deficiency) syndrome in horses. Each mL contains: 5.48 mg sodium selenite (equivalent to 2.5 mg selenium), 50 mg (68 USP units or IU) vitamin E (as d-alpha tocopheryl acetate), 250 mg polyoxyethylated vegetable oil, 2% benzyl alcohol (preservative), water for injection q.s. Sodium hydroxide and/or hydrochloric acid may be added to adjust pH.
-
CLINICAL PHARMACOLOGY
PHARMACOLOGY It has been demonstrated that selenium and tocopherol exert physiological effects and that these effects are intertwined with sulfur metabolism. Additionally, tocopherol appears to have a significant role in the oxidation process, thus suggesting an interrelationship between selenium and tocopherol in overcoming sulfur-induced depletion and restoring normal metabolism. Although oral ingestion of adequate amounts of selenium and tocopherol would seemingly restore normal metabolism, it is apparent that the presence of sulfur and, perhaps, other factors interfere during the digestive process with proper utilization of selenium and tocopherol. When selenium and tocopherol are injected, they bypass the digestive process and exert their full metabolic effects promptly on cell metabolism. Anti-inflammatory action has been demonstrated by selenium-tocopherol in the Selye Pouch Technique and experimentally induced polyarthritis study in rats.
- VETERINARY INDICATIONS
-
SPL UNCLASSIFIED SECTION
CAUTION Intravenous administration, if elected, should be by slow injection.
Emulsions injected intramuscularly into the horse may produce transitory local muscle soreness and can be prevented to some degree by injecting deeply (2 to 21/2 inches), in divided doses, in two or more sites. Do not continue therapy in horses demonstrating such sensitivity.
Selenium is toxic if administered in excess. A fixed dose schedule is therefore important (read package insert for each selenium-tocopherol product carefully before using).
-
WARNINGS
WARNINGS Anaphylactoid reactions, some of which have been fatal, have been reported in horses administered E-SE Injection. Signs include excitement, sweating, trembling, ataxia, respiratory distress, and cardiac dysfunction. These reactions have been reported as associated both with intravenous and intramuscular injections. It is presently unknown whether the mode of application affects the frequency of such reactions. However, reactions associated with intramuscular injections have been reported to manifest more slowly and hence may give more time to institute treatment for anaphylaxis, such as epinephrine and/or corticosteroid injection.
Medications which have been reported to cause major adverse reactions in horses should be avoided when E-SE is administered, unless the condition of the animal requires such use.
Not to be used in horses intended for food.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION Administration: slow intravenous injection (see WARNINGS) or deep intramuscular injections, in divided doses, in two or more sites in the gluteal or cervical muscles. Dosage: 1 mL per 100 pounds of body weight. May be repeated at 5-10 day intervals.
-
PRECAUTIONS
PRECAUTIONS Selenium-Tocopherol Deficiency (STD) syndrome produces a variety and complexity of symptoms often interfering with a proper diagnosis. Even in selenium deficient areas there are other disease conditions which produce similar clinical signs. It is imperative that all these conditions be carefully considered prior to treatment of STD syndrome. Serum selenium levels, elevated SGOT, and creatine levels may serve as aids in arriving at a diagnosis of STD, when associated with other indices.
Important Use only the selenium-tocopherol product recommended for each species. Each formulation is designed for the species indicated to produce the maximum efficacy and safety.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
E-SE
sodium selenite and .alpha.-tocopherol acetate, d- injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0061-0709 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM SELENITE (UNII: HIW548RQ3W) (SELENITE ION - UNII:KXO0259XJ1) SELENIUM 2.5 mg in 1 mL .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 68 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-0709-04 1 in 1 CARTON 1 100 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA030315 07/09/1964 Labeler - Schering Corporation (001317601)