Label: LIDOBAND- lidocaine ring
- NDC Code(s): 86174-101-01, 86174-101-02, 86174-101-03, 86174-101-04
- Packager: Alberta Veterinary Laboratories Ltd
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
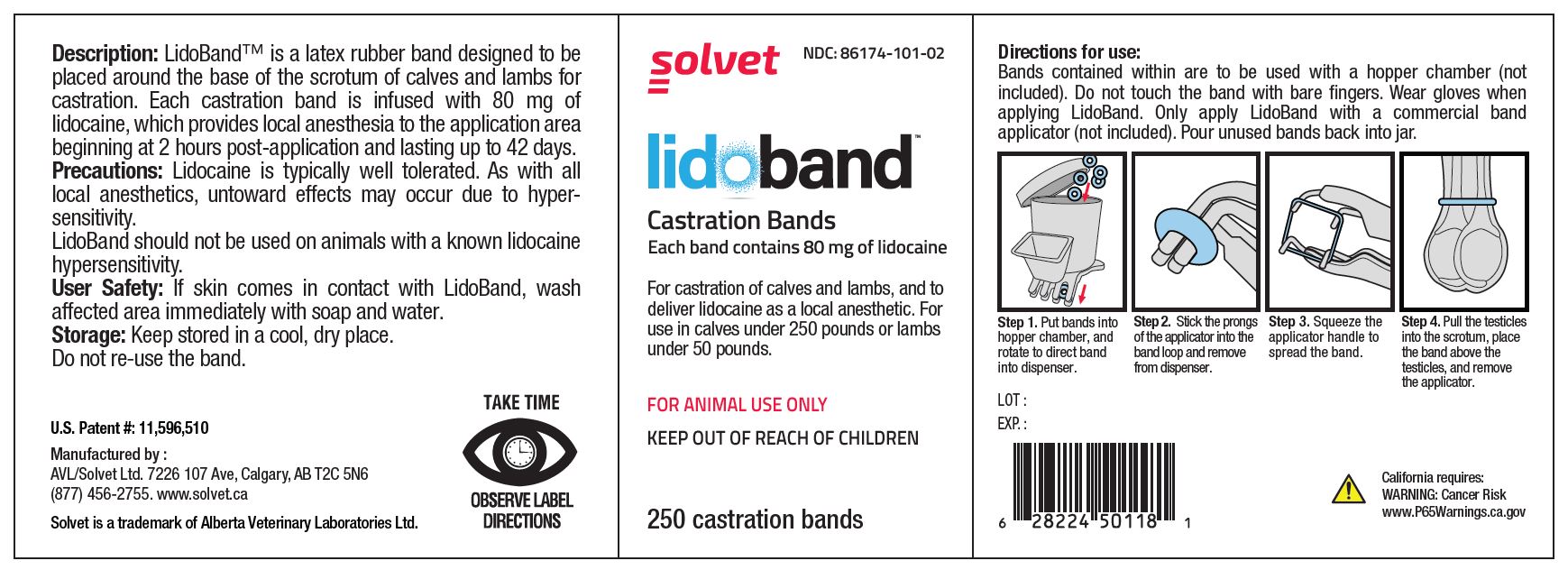
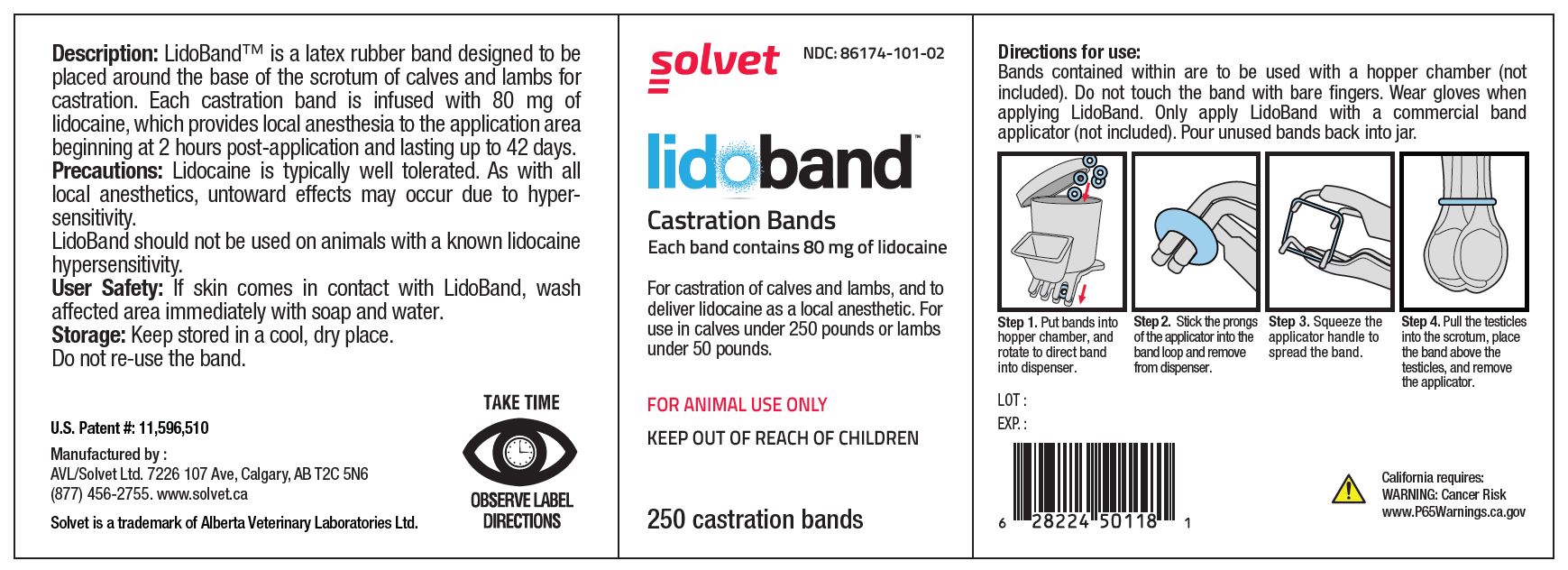
LidoBand Castration Bands
Solvet
LidoBand Castration Bands
Each band contains 80 mg of lidocaine.
For castration of calves and lambs, and to deliver lidocaine as a local anesthetic. For use in calves under 250 pounds or lambs under 50 pounds.
FOR ANIMAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
TAKE TIME OBSERVE LABEL DIRECTIONS
- Description:
- Precautions:
- User Safety:
- Storage:
-
Directions for use: (8, 40, or 80-count box)
Each band is individually packaged in a foil-sealed blister. Do not touch the band with bare fingers. Wear gloves when applying LidoBand. Only apply LidoBand with a commercial band applicator (not included).
Step 1. Puncture the blister surface with the band applicator.
Step 2. Remove the band from packaging while loaded on the prongs of the applicator.
Step 3. Squeeze the applicator handle to spread the band.
Step 4. Pull the testicles into the scrotum, place the band above the testicles, and remove the applicator.
-
Directions for use: (250 count jar)
Bands contained within are to be used with a hopper chamber (not included). Do not touch the band with bare fingers. Wear gloves when applying LidoBand. Only apply LidoBand with a commercial band applicator (not included). Pour unused bands back into jar.
Step 1. Put bands into hopper chamber, and rotate to direct band into dispenser.
Step 2. Stick the prongs of the applicator into the band loop and remove from dispenser.
Step 3. Squeeze the applicator handle to spread the band.
Step 4. Pull the testicles into the scrotum, place the band above the testicles, and remove the applicator.
- Product Labels
-
INGREDIENTS AND APPEARANCE
LIDOBAND
lidocaine ringProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86174-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 80 mg Inactive Ingredients Ingredient Name Strength ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) 30 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86174-101-01 80 in 1 BOX 2 NDC:86174-101-02 250 in 1 JAR 3 NDC:86174-101-03 40 in 1 BOX 4 NDC:86174-101-04 8 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/20/2023 Labeler - Alberta Veterinary Laboratories Ltd (204293518) Registrant - Alberta Veterinary Laboratories Ltd (204293518) Establishment Name Address ID/FEI Business Operations Alberta Veterinary Laboratories Ltd 204293518 manufacture Establishment Name Address ID/FEI Business Operations Swati Spentose Private Limited 650621472 api manufacture