Label: GLYCOPYRROLATE injection, solution
- NDC Code(s): 70121-1698-1, 70121-1698-7, 70121-1699-1, 70121-1699-7
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
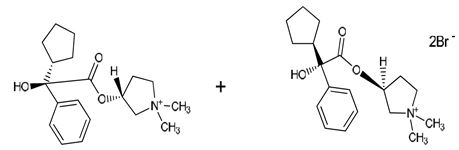
DESCRIPTIONGlycopyrrolate Injection, USP is a synthetic anticholinergic agent. Each 1 mL contains glycopyrrolate, USP 0.2 mg, water for injection, USP q.s., pH adjusted, when necessary, with hydrochloric ...
-
CLINICAL PHARMACOLOGYGlycopyrrolate, like other anticholinergic (antimuscarinic) agents, inhibits the action of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that ...
-
INDICATIONS AND USAGEIn Anesthesia - Glycopyrrolate injection is indicated for use as a preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions; to reduce the volume and free ...
-
CONTRAINDICATIONSKnown hypersensitivity to glycopyrrolate or any of its inactive ingredients. In addition, in the management of peptic ulcer patients, because of the longer duration of therapy, glycopyrrolate ...
-
WARNINGSThis drug should be used with great caution, if at all, in patients with glaucoma. Glycopyrrolate injection may produce drowsiness or blurred vision. The patient should be cautioned regarding ...
-
PRECAUTIONSGeneral - Investigate any tachycardia before giving glycopyrrolate injection since an increase in the heart rate may occur. Use with caution in patients with: coronary artery disease; congestive ...
-
ADVERSE REACTIONSAnticholinergics, including glycopyrrolate injection, can produce certain effects, most of which are extensions of their pharmacologic actions. Adverse reactions may include xerostomia (dry ...
-
OVERDOSAGETo combat peripheral anticholinergic effects, a quaternary ammonium anticholinesterase such as neostigmine methylsulfate (which does not cross the blood-brain barrier) may be given intravenously ...
-
DOSAGE AND ADMINISTRATIONParenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Glycopyrrolate injection may be ...
-
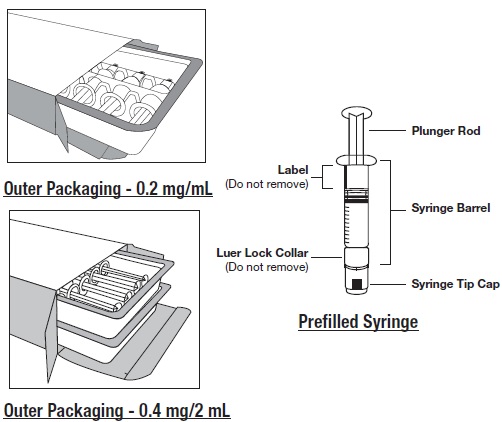
HOW SUPPLIEDGlycopyrrolate Injection USP, 0.2 mg/mL is available as a preservative-free, sterile, clear, colorless solution supplied in prefilled single-dose glass syringes as ...
-
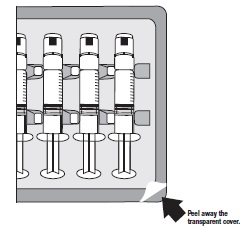
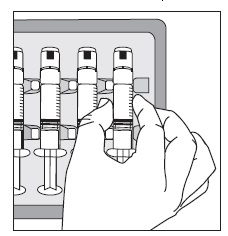
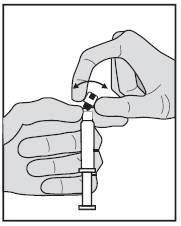
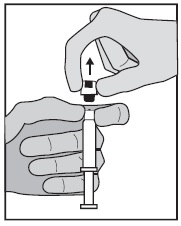
INSTRUCTIONS FOR USEGlycopyrrolate (glye” koe pir’ oh late) Injection, USP - Figure 1: Outer Packaging and Prefilled Syringe - NOTES: - Do not introduce any other fluid into the syringe at any time. - Do not ...
-
PRINCIPAL DISPLAY PANELNDC 70121-1698-1 - Glycopyrrolate Injection USP, 0.2 mg/mL - Rx only - 1 mL Prefilled Single-Dose Syringe Label - Amneal Pharmaceuticals LLC - NDC 70121-1698-7 - Glycopyrrolate Injection USP ...
-
INGREDIENTS AND APPEARANCEProduct Information