Label: DIPHENOXYLATE HYDROCHLORIDE AND ATROPINE SULFATE tablet
- NDC Code(s): 75826-107-00, 75826-107-03, 75826-107-11, 75826-107-33, view more
- Packager: Winder Laboratories LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
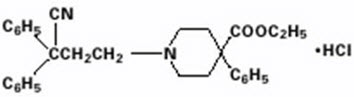
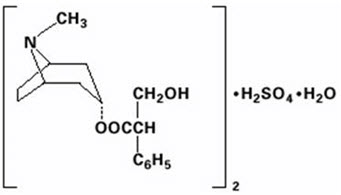
DESCRIPTIONEach diphenoxylate hydrochloride and atropine sulfate tablet, USP contains: 2.5 mg of diphenoxylate hydrochloride USP (equivalent to 2.3 mg of diphenoxylate) and 0.025 mg of atropine sulfate USP ...
-
CLINICAL PHARMACOLOGYDiphenoxylate is rapidly and extensively metabolized in man by ester hydrolysis to diphenoxylic acid (difenoxine), which is biologically active and the major metabolite in the blood. After a 5-mg ...
-
INDICATIONS AND USAGEDiphenoxylate hydrochloride and atropine sulfate is indicated as adjunctive therapy in the management of diarrhea in patients 13 years of age and older.
-
CONTRAINDICATIONSDiphenoxylate hydrochloride and atropine sulfate is contraindicated in: Pediatric patients less than 6 years of age due to the risks of respiratory and central nervous system (CNS) depression ...
-
WARNINGSRespiratory and/or CNS Depression in Pediatric Patients Less Than 6 Years of Age - Cases of severe respiratory depression and coma, leading to permanent brain damage or death have been reported ...
-
PRECAUTIONSAtropinism - Since a subtherapeutic dose of atropine has been added to Diphenoxylate hydrochloride and atropine sulfate, consideration should be given to the development of adverse reactions ...
-
ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in labeling: Respiratory and/or CNS depression (see - WARNINGS) Anticholinergic and opioid-toxicities, including ...
-
DRUG ABUSE AND DEPENDENCEControlled substance - Diphenoxylate hydrochloride and atropine sulfate is classified as a Schedule V controlled substance by federal regulation. Diphenoxylate hydrochloride is chemically related ...
-
OVERDOSAGEDiagnosis - Overdosage can be life-threatening. Symptoms of overdosage may include opioid and/or anticholinergic effects including respiratory depression, coma, delirium, lethargy, dryness of the ...
-
DOSAGE AND ADMINISTRATIONManagement of Diarrhea in Patients 13 Years of Age and Older - Diphenoxylate hydrochloride and atropine sulfate is recommended as adjunctive therapy for the management of diarrhea in patients 13 ...
-
HOW SUPPLIEDDiphenoxylate Hydrochloride and Atropine Sulfate Tablets, USP are available containing 2.5 mg of diphenoxylate hydrochloride, USP and 0.025 mg of atropine sulfate, USP. Tablets– white to off ...
-
SPL UNCLASSIFIED SECTIONThis product's label may have been updated. For current full prescribing information, please visit www.winderlabs.com. Manufactured By: Winder Laboratories, LLC - Winder GA, 30680 ...
-
PRINCIPAL DISPLAY PANEL - 1000 Tablet Bottle LabelDiphenoxylate Hydrochloride and Atropine Sulfate - Tablets, USP CV - 2.5 mg / 0.025 mg - Rx Only - 1000 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information