Label: PENCICLOVIR cream
- NDC Code(s): 45802-440-75
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PENCICLOVIR CREAM safely and effectively. See full prescribing information for PENCICLOVIR CREAM. PENCICLOVIR cream, for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Penciclovir Cream, 1% is a deoxynucleoside analog HSV DNA polymerase inhibitor indicated for the treatment of recurrent herpes labialis (cold sores) in adults and children 12 years of age or ...
-
2 DOSAGE AND ADMINISTRATION Apply Penciclovir Cream, 1% every 2 hours during waking hours for a period of 4 days. Start treatment as early as possible (i.e., during the prodrome or when lesions appear).
-
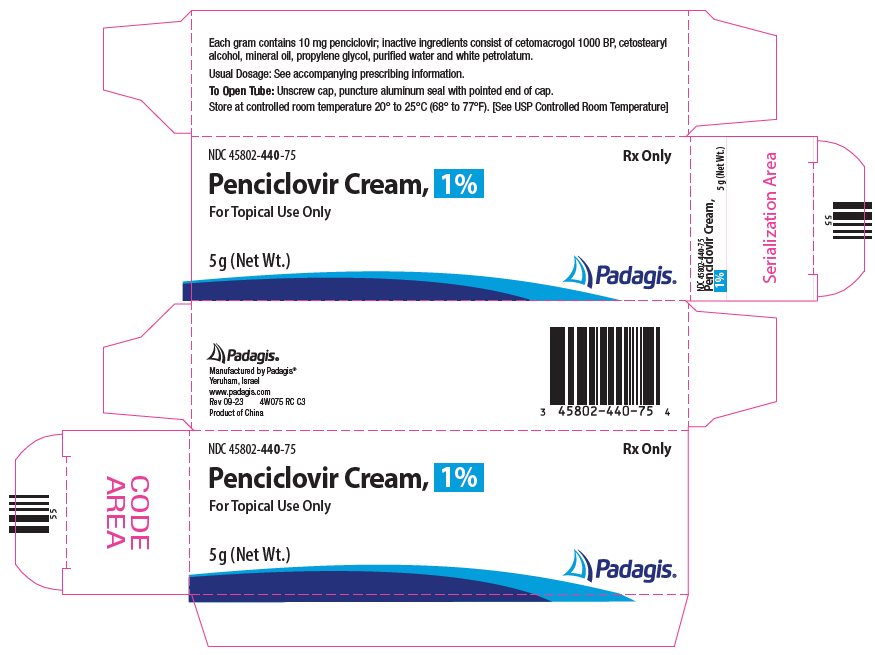
3 DOSAGE FORMS AND STRENGTHS Penciclovir Cream, 1% is supplied in a 5 gram tube containing 10 mg of penciclovir per gram in a cream base, which is equivalent to 1% (w/w).
-
4 CONTRAINDICATIONS Penciclovir Cream, 1% is contraindicated in patients with known hypersensitivity to the product or any of its components.
-
5 WARNINGS AND PRECAUTIONS 5.1 General - Penciclovir Cream, 1% should only be used on herpes labialis on the lips and face. Because no data are available, application to human mucous membranes is not recommended ...
-
6 ADVERSE REACTIONS 6.1 Clinical Studies - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to ...
-
7 DRUG INTERACTIONS No drug interaction studies have been performed with Penciclovir Cream, 1%. Due to minimal systemic absorption of Penciclovir Cream, 1%, systemic drug interactions are unlikely.
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Penciclovir Cream, 1% is not absorbed systemically following topical administration and maternal use is not expected to result in fetal exposure to the ...
-
10 OVERDOSAGE Since penciclovir is poorly absorbed following oral administration, adverse reactions related to penciclovir ingestion are unlikely. There is no information on overdose.
-
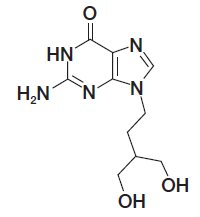
11 DESCRIPTION Penciclovir Cream, 1% contains penciclovir, an antiviral agent active against herpes viruses. Penciclovir Cream, 1% is available for topical administration as a 1% white cream. Each gram of ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Penciclovir is an antiviral agent active against alpha herpes viruses [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Measurable penciclovir concentrations were ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In clinical trials, systemic drug exposure following topical administration of penciclovir cream was negligible, as the penciclovir ...
-
14 CLINICAL STUDIES Penciclovir cream was studied in two double-blind, placebo (vehicle)-controlled trials for the treatment of recurrent herpes labialis in which otherwise healthy adults were randomized to either ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Penciclovir Cream, 1% is supplied in a 5 gram tube containing 10 mg of penciclovir per gram in a cream base, which is equivalent to 1% (w/w). The white cream is available as follows: NDC ...
-
17 PATIENT COUNSELING INFORMATION Administration Instructions - Advise the patient that treatment should be started at the earliest sign of a cold sore (i.e. tingling, redness, itching, or bump). Inform the patient to apply the ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 45802-440-75 - Penciclovir Cream, 1% For Topical Use Only - 5 g (Net Wt.)
-
INGREDIENTS AND APPEARANCEProduct Information