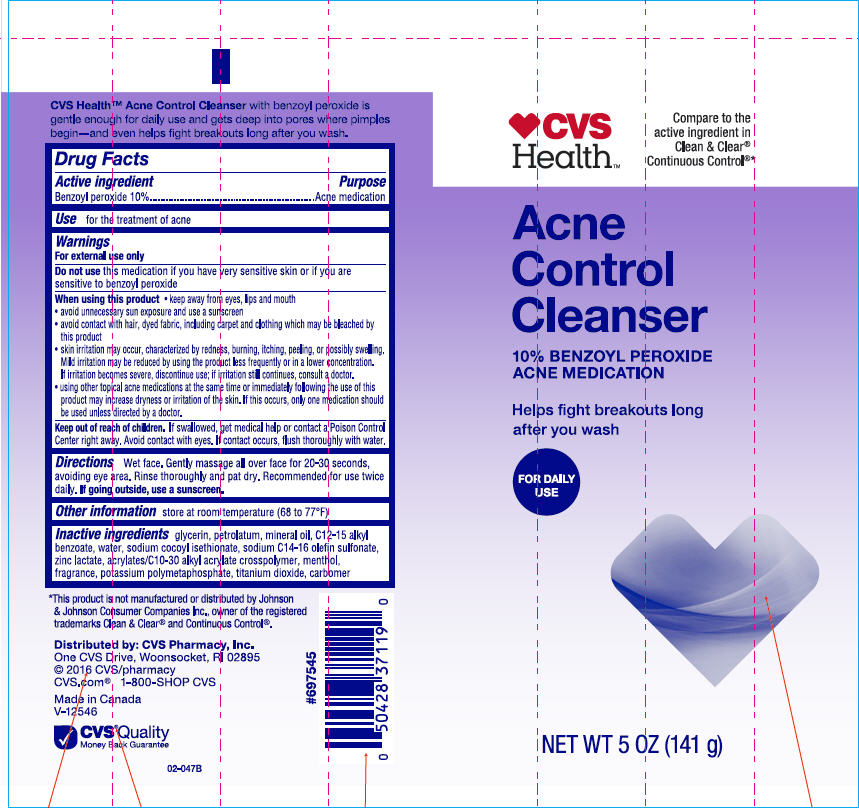
Label: CVS HEALTH ACNE CONTROL CLEANSER ACNE MEDICATION- benzoyl peroxide lotion
- NDC Code(s): 69842-571-02
- Packager: CVS Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use this medication if you have very sensitive skin or if you are sensitive to benzoyl peroxide.
When using this product keep away from eyes, lips and mouth
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with hair or dyed fabric, including carpet and clothing which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Mild irritation may be reduced by using the product less frequently or in a lower concentration. If irritation becomes severe, discontinue use; if irritation still continues, consult a doctor.
- using other topical acne medication at the same time or immediately following the use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
- Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 141 g Tube Label
-
INGREDIENTS AND APPEARANCE
CVS HEALTH ACNE CONTROL CLEANSER ACNE MEDICATION
benzoyl peroxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-571 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzoyl Peroxide (UNII: W9WZN9A0GM) (Benzoyl Peroxide - UNII:W9WZN9A0GM) Benzoyl Peroxide 100 mg in 1 g Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) Petrolatum (UNII: 4T6H12BN9U) Mineral Oil (UNII: T5L8T28FGP) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Water (UNII: 059QF0KO0R) Sodium Cocoyl Isethionate (UNII: 518XTE8493) Sodium C14-16 Olefin Sulfonate (UNII: O9W3D3YF5U) Zinc Lactate (UNII: 2GXR25858Y) Carbomer Interpolymer Type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Menthol, Unspecified Form (UNII: L7T10EIP3A) Potassium Metaphosphate (UNII: 01DMT14Z63) Titanium Dioxide (UNII: 15FIX9V2JP) Carbomer Homopolymer Type C (Allyl Pentaerythritol Crosslinked) (UNII: 4Q93RCW27E) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-571-02 141 g in 1 TUBE; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/01/2014 Labeler - CVS Health (062312574) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations Garcoa, Inc. 036464697 MANUFACTURE(69842-571)