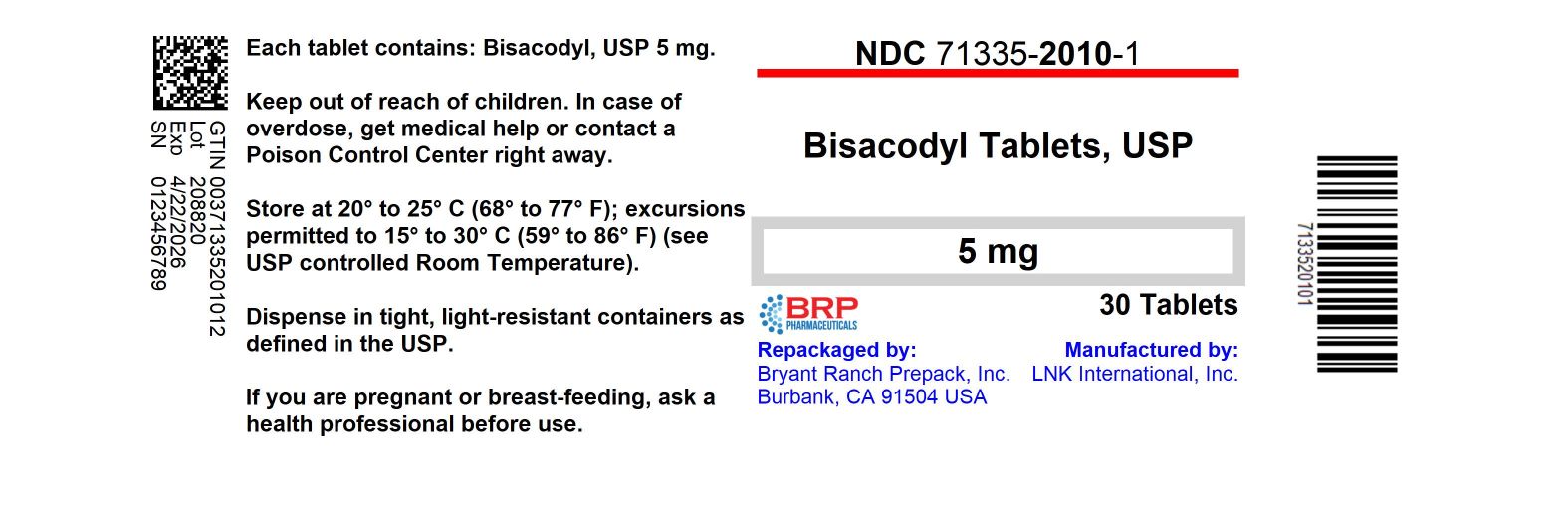
Label: BISACODYL tablet, delayed release
- NDC Code(s): 71335-2010-0, 71335-2010-1, 71335-2010-2, 71335-2010-3, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0904-6748
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each tablet)
Bisacodyl USP, 5 mg
-
Purpose
Stimulant laxative
-
Uses
for relief of occasional constipation and irregularity - this product generally produces bowel movement in 6 to 12 hours
-
Warnings
Do not use - if you cannot swallow without chewing. Ask a doctor before use if you have - stomach pain, nausea or vomiting - a sudden change in bowel habits that lasts more than 2 ...
-
Directions
do not take more than directed - take with a glass of water - adults and children 12 years and overtake 1 to 3 tablets in a single daily dose - children 6 to under 12 yearstake 1 tablet in a ...
-
Other information
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN - store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F) avoid excessive humidity - see end flap ...
-
Inactive ingredients
acacia, ammonium hydroxide, calcium carbonate, carnauba wax, colloidal anhydrous silica, corn starch, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black ...
-
Questions or comments?
1-800-426-9391
-
HOW SUPPLIED
Bisacodyl USP, 5 mg - NDC: 71335-2010-0: 25 Tablets in a BOTTLE - NDC: 71335-2010-1: 30 Tablets in a BOTTLE - NDC: 71335-2010-2: 2 Tablets in a BOTTLE - NDC: 71335-2010-3: 3 Tablets in a BOTTLE - NDC ...
-
PRINCIPAL DISPLAY PANELBisacodyl 5mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information