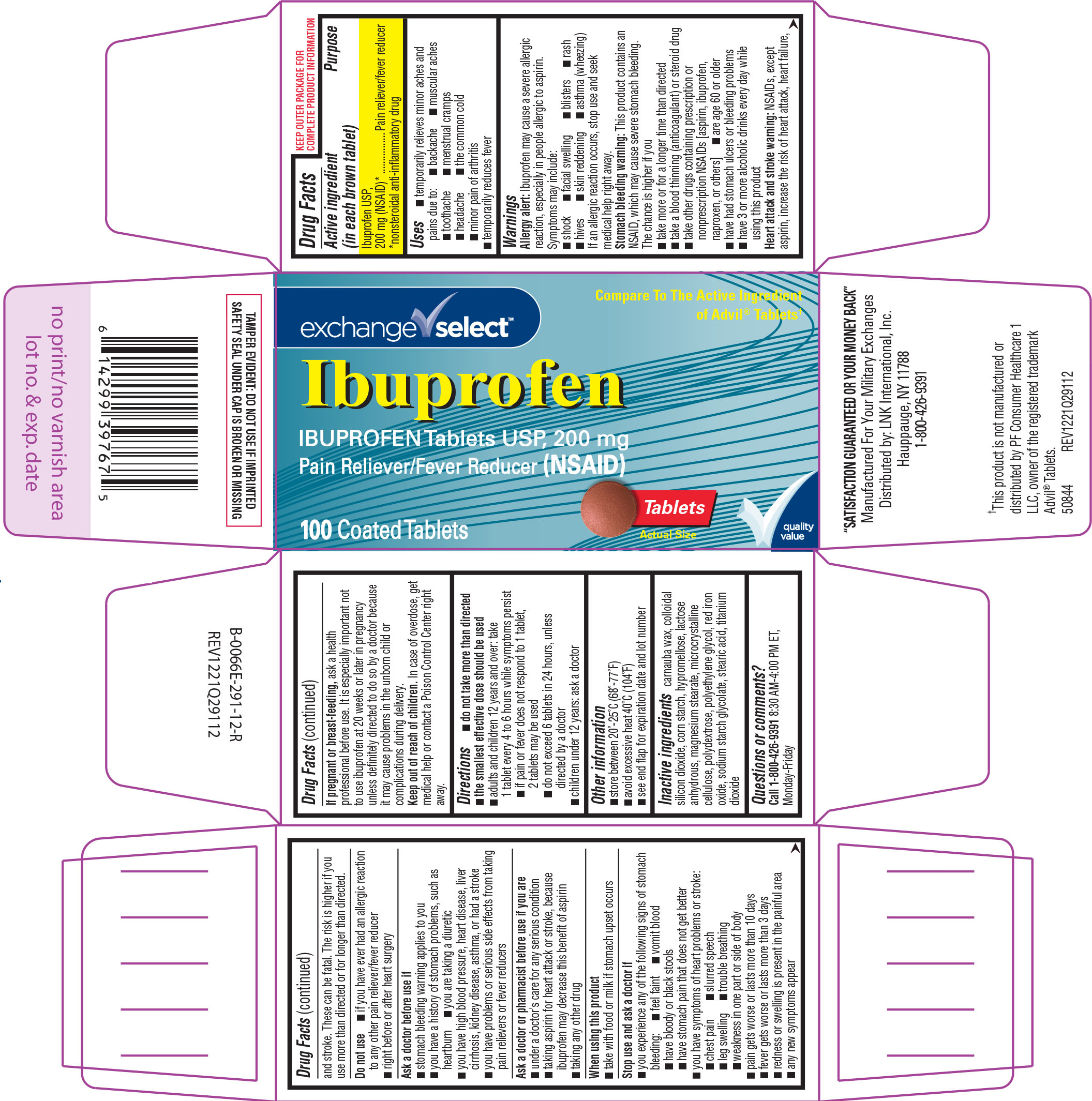
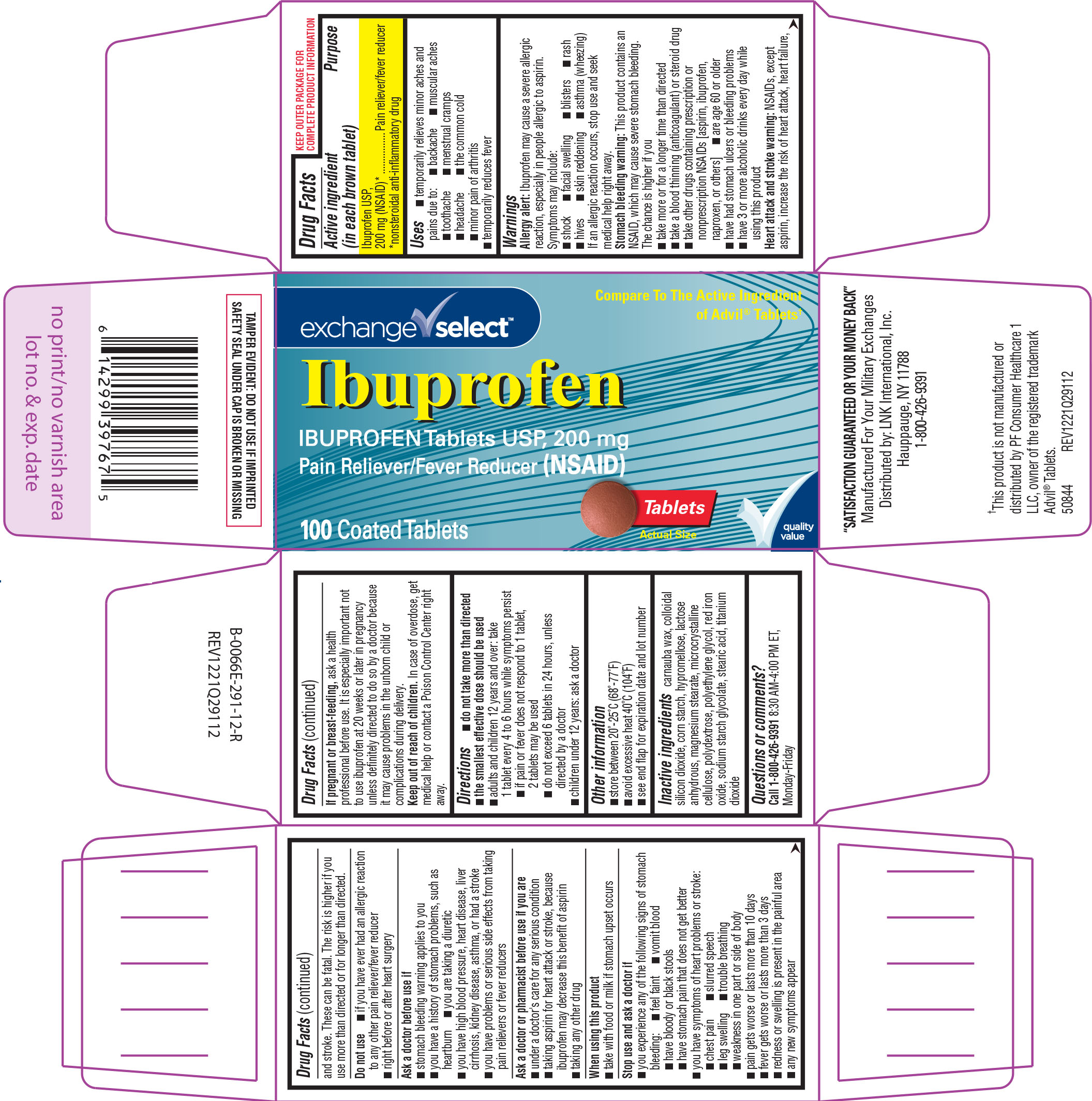
Label: IBUPROFEN tablet, film coated
- NDC Code(s): 55301-291-03, 55301-291-06, 55301-291-12, 55301-291-14
- Packager: ARMY AND AIR FORCE EXCHANGE SERVICE
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 2, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each brown tablet)
Ibuprofen USP, 200 mg (NSAID)* *nonsteroidal anti-inflammatory drug
-
Purpose
Pain reliever/fever reducer
-
Uses
temporarily relieves minor aches and pains due to: menstrual cramps - toothache - backache - the common cold - headache - muscular aches - minor pain of arthritis - temporarily reduces fever
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: facial swelling - shock - asthma (wheezing) skin ...
-
Directions
do not take more than directed - the smallest effective dose should be used - adults and children 12 years and over: take 1 tablet every 4 to 6 hours while symptoms persist - if pain or fever does ...
-
Other information
store between 20º-25ºC (68º-77ºF) avoid excessive heat 40ºC (104ºF) see end flap for expiration date and lot number
-
Inactive ingredients
carnauba wax, colloidal silicon dioxide, corn starch, hypromellose, lactose anhydrous, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, red iron oxide, sodium ...
-
Questions or comments?
Call 1-800-426-9391 8:30 AM-4:00 PM ET, Monday-Friday
-
Principal Display Panel
exchange ✓ select™ Compare To The Active Ingredient - of Advil® Tablets† Ibuprofen - IBUPROFEN Tablets USP, 200 mg - Pain Reliever/Fever Reducer (NSAID) 100 Coated Tablets - Tablets - Actual ...
-
INGREDIENTS AND APPEARANCEProduct Information