Label: SOLIFENACIN SUCCINATE tablet, film coated
- NDC Code(s): 71205-583-30, 71205-583-60, 71205-583-90
- Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 60505-4703
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOLIFENACIN SUCCINATE TABLETS safely and effectively. See full prescribing information for SOLIFENACIN SUCCINATE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESolifenacin Succinate Tablets are indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended oral dose of Solifenacin Succinate Tablets is 5 mg once daily. If the 5 mg dose is well tolerated, the dose may be increased to 10 mg once ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: •5 mg: round, light yellow, debossed with 23 - •10 mg: round, light pink, debossed with 24
-
4 CONTRAINDICATIONSSolifenacin Succinate Tablets are contraindicated in patients: •With urinary retention [see Warnings and Precautions ( 5.2)] ...
-
5 WARNINGS AND PRECAUTIONS5.1 Angioedema and Anaphylactic Reactions - Angioedema of the face, lips, tongue, and/or larynx have been reported with solifenacin succinate. In some cases, angioedema occurred after the first ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Strong CYP3A4 Inhibitors - Solifenacin is a substrate of CYP3A4. Concomitant use of ketoconazole, a strong CYP3A4 inhibitor, significantly increased the exposure of solifenacin ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no studies with the use of solifenacin succinate in pregnant women to inform a drug-associated risk of major birth defects, miscarriages, or adverse ...
-
10 OVERDOSAGEOverdosage with Solifenacin Succinate Tablets can potentially result in severe antimuscarinic effects and should be treated accordingly. The highest dose ingested in an accidental overdose of ...
-
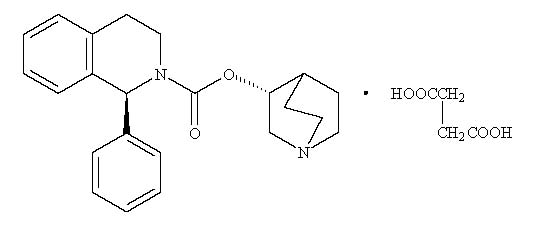
11 DESCRIPTIONSolifenacin Succinate Tablets are a muscarinic receptor antagonist. Chemically, solifenacin succinate is a butanedioic acid compound with (1 S)-(3 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Solifenacin is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergically mediated functions, including ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No increase in tumors was found following the administration of solifenacin succinate to male and female mice for 104 weeks at doses up ...
-
14 CLINICAL STUDIESSolifenacin Succinate Tablets were evaluated in four twelve-week, double-blind, randomized, placebo-controlled, parallel group, multicenter clinical trials for the treatment of overactive bladder ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSolifenacin Succinate Tablets are supplied as round, film-coated tablets, available in bottles as follows: Each 10 mg tablet is light pink and debossed with and “24” on one side and is available ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Angioedema and Anaphylactic Reactions - Inform patients that angioedema and anaphylactic reactions have been ...
-
PATIENT PACKAGE INSERTPatient Information - Solifenacin Succinate - (sol i fen′ a cin sux’ i nate) Tablet - Read the Patient Information that comes with Solifenacin Succinate Tablets before you start taking ...
-
PRINCIPAL DISPLAY PANEL - 10 mg Bottle30 tablets NDC 71205-583-30 - Solifenacin Succinate - Tablets - 10 mg - ONCE-DAILY - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information